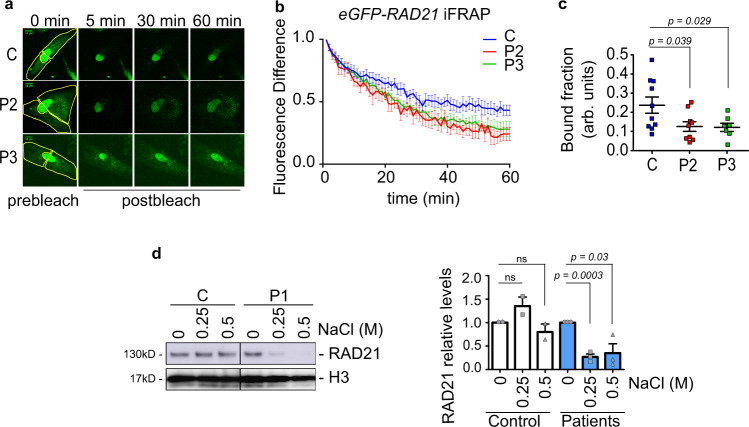
Fig. 2. Chromatin-bound cohesin is less stable in CdLS-derived cells.
a Representatives images of an iFRAP experiment of control (C) and two CdLS patients (P2–3) cells before (0 min) and 5, 30, and 60 min after photobleaching (green channel). The entire cell, except for a small nuclear region, was photobleached (yellow line) and the fluorescence of EGFP-RAD21 redistribution in the bleached and unbleached regions was followed by time-lapse microscopy. b Fluorescence recovery after bleaching was quantified as the difference between bleached and unbleached nuclear regions over 1 h in one control (C, blue) and two CdLS patients (P2, red; P3, green) and normalized to the first post-bleach frame after 2 min. Means and SEMs are shown. Control, n = 10; P2, n = 8; P3, n = 7 cells examined over more than 3 biologically independent experiments. Two-sided unpaired student’s t-test. c The drop in mean fluorescence intensity in the unbleached region at the first post-bleach frame was used to calculate the chromatin-bound fraction. Same cells than in (b) were used. Means and SEMs are shown. Two-sided unpaired student’s t-test. d Chromatin fractions were prepared from cells derived from control (C) and CdLS patient (P) derived cells and washed with 0.25 and 0.5 M of NaCl containing buffer for 30 min. The amount of RAD21 bound to the chromatin was analyzed by immunoblotting. A representative experiment is shown (left panel), in which histone3 (H3) was used as a loading control. Plots of RAD21 levels in controls (white) and 3 biologically independent samples (CdLS patients P1, P2, and P3, blue) are depicted (right panel). Means and SEMs are shown. Two-sided unpaired student’s t-test.