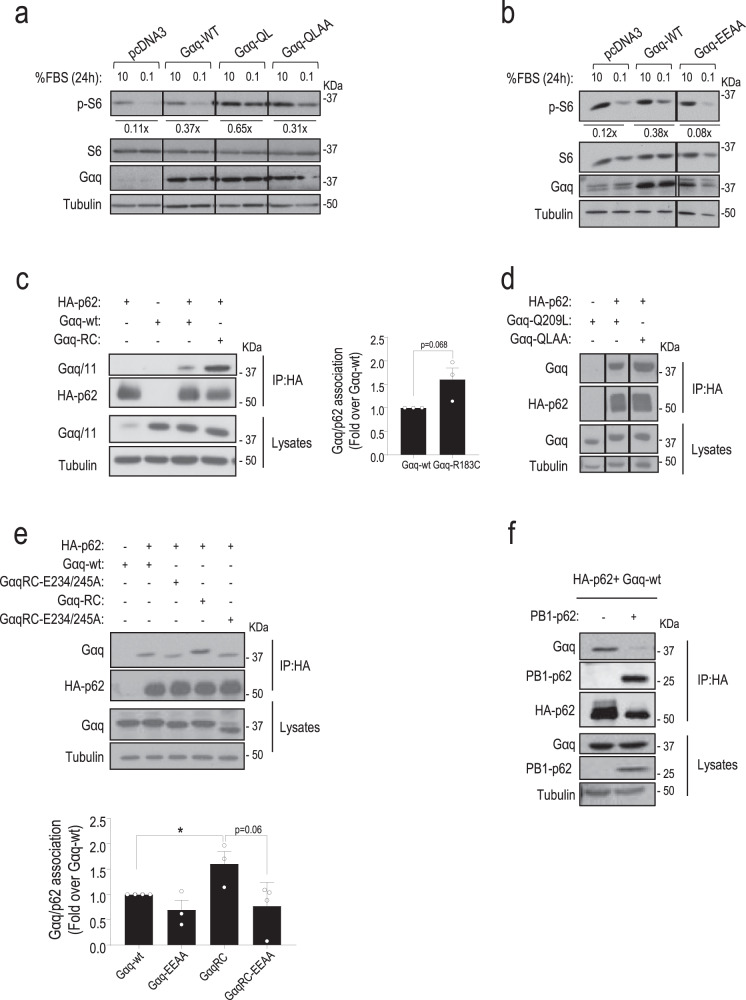
Fig. 6. Gαq participates in the activation mechanism of mTORC1 via noncanonical effectors and associates with p62 through a PB1-like interaction.
a, b DREADD-Gq HEK-293 (a) or CHO cells (b) transiently overexpressing Gαq wt or the indicated Gαq mutants (constitutively active Gq-Q209L, active but PLC-β activation-defective Gq-Q209L/R256A/T257A-GqQL-AA, mutant defective in binding to PB1-domain-containing proteins Gq-EEAA) were starved or not with 0.1% FBS for 24 h. The dephosphorylation fold of S6 upon serum deprivation was calculated with respect to the 10% FBS control of each cell population. A representative blot of three independent experiments is shown. c–f CHO cells were transiently transfected with the indicated combinations of HA-p62, Gαq WT or the indicated Gαq mutants (constitutively active Gq-Q209L or GqRC, active but PLC-β activation-defective GqQL-AA, defective in binding to PB1-domain-containing proteins Gq-EEAA and constitutively active GqRC-EEAA), or the PB1 domain of p62 (GFP-Flag-PB1-p62). In all cases (c–f), co-immunoprecipitation assays and expression controls in lysates were performed as described under “Methods”. Representative blots of three independent experiments are shown. e Data (mean ± SEM of three (Gq-EEAA and GqRC) or four (Gαq WT and GqRC-EEAA) independent experiments) were normalized with total HA-p62 and expressed as fold induction of Gαq/p62 association over control. Statistical significance was analyzed using two-sided unpaired t-test. Gαq WT vs. GqRC, P value = 0.0312. Source data are provided as a Source Data file.