Figure 1.
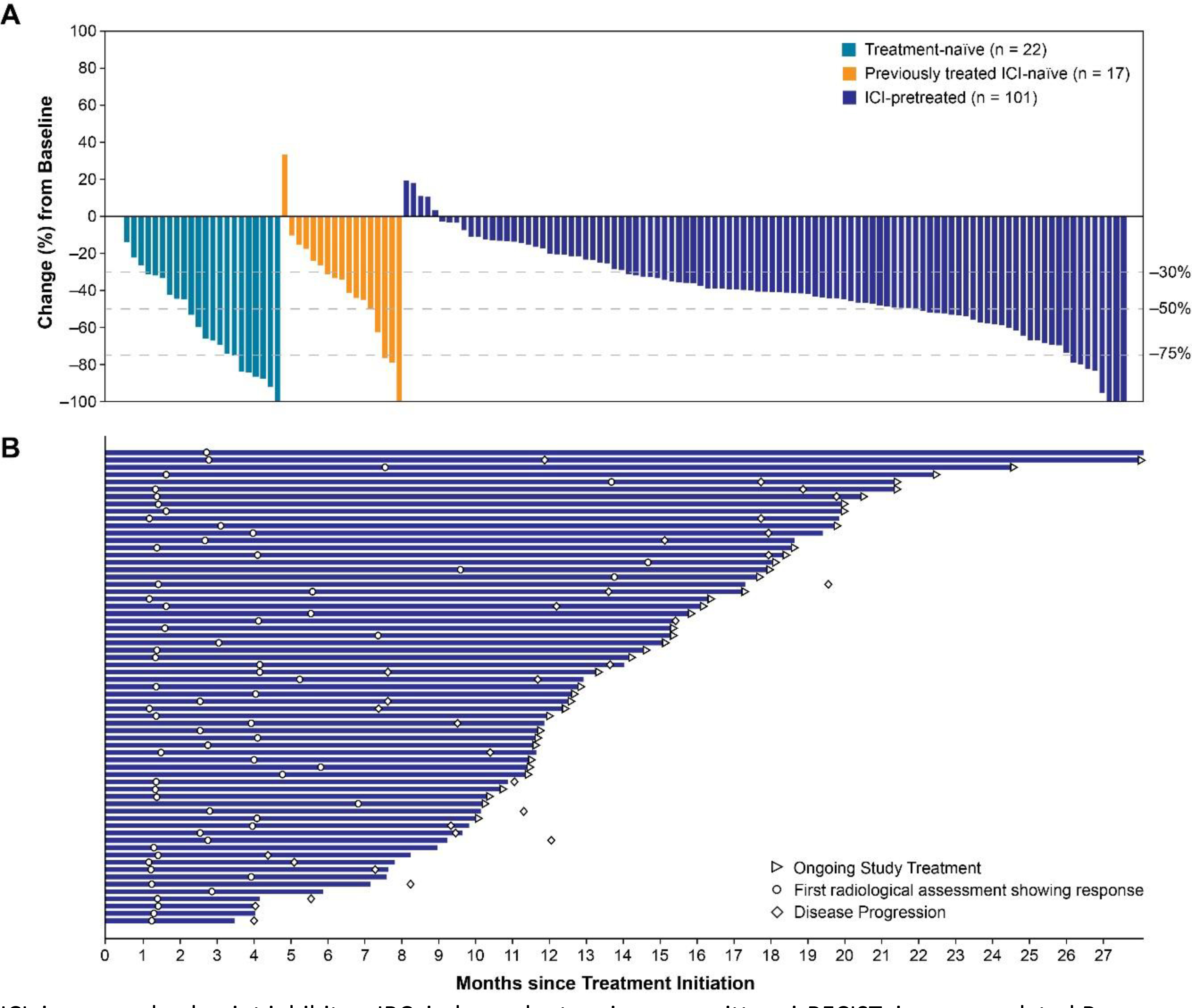
Percentage Change in Sums of Diameters of Target Lesions From Baseline to Nadir (A) and Treatment Durations in ICI-Pretreated Patients Achieving an Objective Response (B) by Investigator Assessment per irRECIST
ICI, immune checkpoint inhibitor; IRC, independent review committee; irRECIST, immune-related Response Evaluation Criteria In Solid Tumors; n, number of patients who had baseline target lesion measurement and at least one postbaseline target lesion measurement by investigator assessment per irRECIST.
