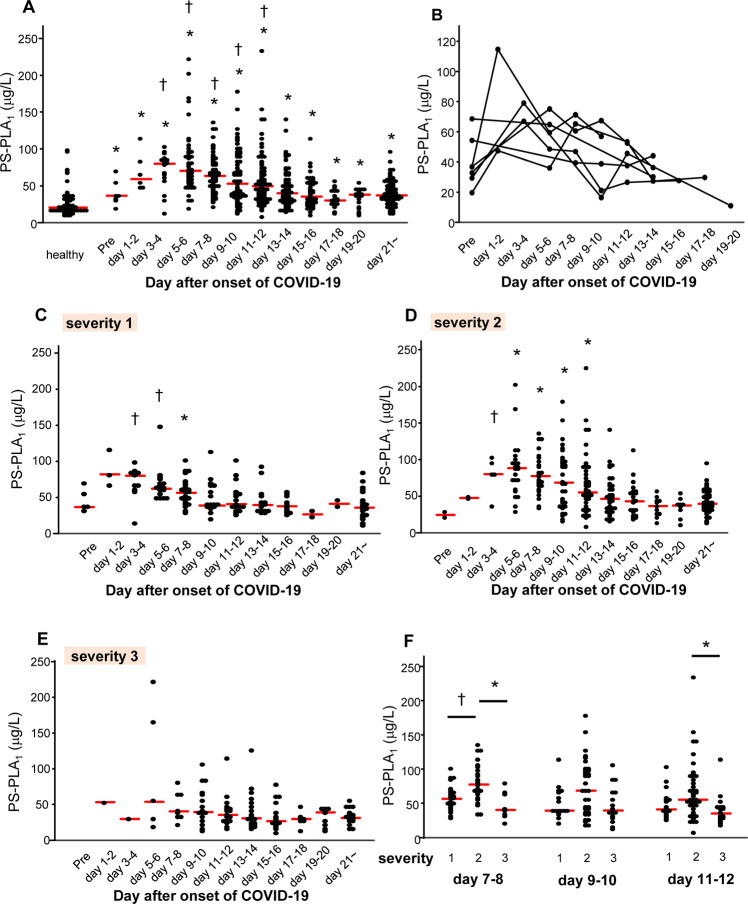
Fig. 1.
Serum PS-PLA1 levels in subjects with COVID-19. The serum PS-PLA1 levels were measured in patients with COVID-19 (severity level 1, n = 41; severity level 2, n = 62; severity level 3, n = 24) and healthy subjects (n = 58). A Time course of serum PS-PLA1 levels in symptomatic COVID-19 patients and distribution of serum PS-PLA1 levels in healthy subjects. The differences in the levels between the healthy subjects and COVID-19 patients were assessed by the Mann–Whitney U test, *P < 0.01 vs. healthy subjects. Differences between the serum PS-PLA1 levels measured on specified days after the onset of COVID-19 symptoms and those measured after day 21 from symptom onset in individual subjects were assessed by the Wilcoxon signed-rank sum test, †P 0.01 vs. level measured after day 21. B Time course of the serum PS-PLA1 levels in the COVID-19 patients for whom samples collected before disease onset (Pre) were available (n = 7). C–F Time course of serum PS-PLA1 levels in patients with mild (C), moderate (D), and severe COVID-19 (E). *P < 0.01; †P < 0.05 vs. level measured after day 21 from symptom onset. F Differences in the serum PS-PLA1 levels on days 7–8, days 9–10, and days 11–12. The differences were assessed using an independent Kruskal–Wallis test, followed by the Games Howell test for post hoc analysis. *P < 0.01; †P < 0.05. The horizontal bars represent the means of independent samples