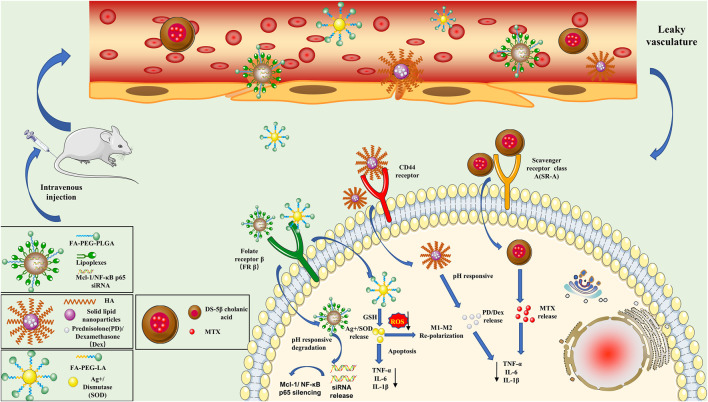
FIGURE 3.
Schematic illustration of the active targeting nanoparticles (NPs) approach in rheumatoid arthritis. (1) Mcl-1/NF-κB p65 siRNA-NPs are fabricated by encapsulating poly-siRNA into lipid–polymer hybrid NPs. Following systemic administration, NPs accumulate in the inflamed joints by taking advantage of the leaky blood vessels and selectively delivering Mcl-1/NF-κB p65 siRNA into activated macrophages through folate receptor–mediated endocytosis. (2) Ag+/SOD-loaded NPs dissolve and release Ag+/SOD in response to intracellular GSH, which synergistically induces apoptosis in M1 macrophages and scavenges ROS to cause the polarization of M1 macrophages to the M2 phenotype in inflamed synovial joints. (3) PD/Dex is wrapped in HA-coated solid lipid NPs to prepare NPs for pH-responsive drug release. HA coating allows NPs to enter macrophages through CD44 receptor–mediated endocytosis, thereby reducing the release of TNF-α, IL-1β, and IL-6. (4) Dextran sulfate acts as a ligand for macrophage scavenger receptor class A, which is overexpressed by activated macrophages. Nanoparticles enter activated macrophages to release MTX and inhibit the release of TNF-α, IL-1β, and IL-6. Dex, dexamethasone; FA-PEG-PLGA, folic acid-poly (ethylene glycol)-poly (lactide-co-glycolide); FA-PEG-LA, FA-PEG-lipoic acid; GSH, glutathione; HA, hyaluronan; IL-1β, interleukin-1β; IL-6, interleukin-6; Mcl-1, myeloid cell leukemia-1; MTX, methotrexate; NF-κB, nuclear factor-κB; PD, prednisolone; ROS, reactive oxygen species; siRNA, small interfering RNA; SOD, superoxide dismutase; TNF-α, tumor necrosis factor α.