Abstract
Primary malignant brain tumors are a major cause of morbidity and mortality in both adults and children, with a dismal prognosis despite multimodal therapeutic approaches. In the last years, a specific subpopulation of cells within the tumor bulk, named cancer stem cells (CSCs) or tumor-initiating cells, have been identified in brain tumors as responsible for cancer growth and disease progression. Stemness features of tumor cells strongly affect treatment response, leading to the escape from conventional therapeutic approaches and subsequently causing tumor relapse. Recent research efforts have focused at identifying new therapeutic strategies capable of specifically targeting CSCs in cancers by taking into consideration their complex nature. Aberrant epigenetic machinery plays a key role in the genesis and progression of brain tumors as well as inducing CSC reprogramming and preserving CSC characteristics. Thus, reverting the cancer epigenome can be considered a promising therapeutic strategy. Three main epigenetic mechanisms have been described: DNA methylation, histone modifications, and non-coding RNA, particularly microRNAs. Each of these mechanisms has been proven to be targetable by chemical compounds, known as epigenetic-based drugs or epidrugs, that specifically target epigenetic marks. We review here recent advances in the study of epigenetic modulators promoting and sustaining brain tumor stem-like cells. We focus on their potential role in cancer therapy.
Keywords: Cancer stem cells, Epigenetics, Brain tumors, Epigenetic drugs, Histone deacetylase inhibitors, DNA methyltransferase inhibitors
Core Tip: Cancer stem cells (CSCs) are characterized by an altered epigenome that contributes to treatment failure and tumor relapse. Physicians are looking for new therapeutic approaches to target specifically CSCs in cancers. In this review, we summarize literature data about epigenetic markers in brain CSCs and shed light on new epigenetic therapies.
INTRODUCTION
Primary malignant brain tumors are a heterogeneous group of tumors arising from the brain parenchyma and its surrounding structures. They represent a major cause of morbidity and mortality in both adults and children. In particular, brain tumors are the most frequently reported solid malignancies in children, accounting for up to 20% of childhood cancers[1]. Brain cancers require a multimodal treatment that includes surgical resection, chemotherapy, and radiation therapy[2]. This therapeutic strategy includes a particularly invasive surgery as well as brain irradiation and can lead to long-lasting side effects, significantly decreasing the patient’s quality of life.
Brain tumors can harbor genetic and epigenetic alterations that make them resistant to conventional pharmacological treatment[3]. Current therapeutic approaches do not consider the presence of a specific subpopulation of cells within the tumor bulk, known as cancer stem cells (CSCs) or tumor-initiating cells. These cells are not responsive to conventional treatment, promoting tumor relapse[4]. In this scenario, it is of fundamental importance to seek alternative therapeutic strategies that take into account the genetic and epigenetic alterations of the tumor as well as the presence of tumor-initiating cells. Nowadays, to address this challenge, the epigenetic profile of CSCs is being thoroughly studied and several epigenetics drugs are being tested in in vitro and in vivo studies on CSC cultures[5].
This review aims to summarize and organize the current epigenetics strategies for eradicating brain CSCs based on CSC epigenetic modulators.
Brain cancer stem cells
CSCs are considered the “reservoirs” of the tumor. They are defined as a small subset of stem cells capable of proliferating and generating diverse and heterogeneous cell types that constitute the whole tumor[6]. CSCs reside in specific anatomical areas called “niches”, where they interact with the microenvironment surrounding them[7]. Like their normal tissue counterpart, CSCs exhibit “stem-like” characteristics: (1) Cell quiescence: A way to preserve self-renewal and to avoid genetic perturbation that could occur during cell division; (2) Self-renewal capacity: The ability to proliferate symmetrically and asymmetrically; (3) Multipotency: The ability to give rise to heterogeneous cells with different proliferative potential; (4) Migration: The ability to migrate and disseminate; (5) Tissue regeneration: The ability to give rise to new tumoral tissues; and (6) Communication: The ability to interact with the microenvironment.
The substantial difference between CSCs and normal tissue stem cells is that proliferation/death signals are aberrant and dysregulated in cancer, whereas normal tissue stem cells can maintain physiological homeostasis.
CSCs were discovered for the first time in acute myeloid leukemia[8]. Subsequently, this tumor-initiation subpopulation was also identified in many solid tumors, including brain cancers[9,10]. CSCs have been discovered and isolated in major brain tumors [e.g., medulloblastoma (MB), gliomas, ependymoma][11-13] from both pediatric and adult patients[14-16].
In the first instance, cell surface markers were used to identify this subset of tumor-initiating cells. The cell surface antigen CD133 has been most frequently used to mark CSCs in various solid tumors[17]. In the context of the brain, Singh et al[11] demonstrated that CD133+ human brain tumor cells were able to initiate and recapitulate the original tumor in in vivo mouse models. Further study demonstrated that a high expression level of CD133 was correlated with poor prognosis in brain cancer patients, reinforcing CD133’s role as a “brain stemness” marker[18].
The accuracy of this detection method remains very controversial because cell surface markers evolve rapidly in response to different environmental stimuli, disease states, and tumor progression[19,20]. Moreover, brain CSCs and neural stem cells share common phenotypic markers (e.g., CD133, CD15, CD44)[21]. Hence, the marker-dependent identification method alone is insufficient to discriminate correctly CSCs from normal tissue ones. Recent studies[22-24] investigate complementary methods that consider cell dysregulated survival pathways and genetic and epigenetic signatures. As already described by Abbaszadegan et al[23], the gold standard strategy to identify efficiently brain CSCs is to test their in vivo tumorigenicity. Limiting dilution assay is the best tumorigenicity method that is commonly used for evaluation of CSCs frequency. However, this method presents some critical points, being influenced by the number of the cells, the implantation site, and growing time of incubation. Moreover, it is not feasible on large scale studies. Complementary in vitro functional assays could be used to identify CSCs based on: (1) Their intrinsic properties (e.g., self-renewal, asymmetrical division, slow proliferation phenotype, and aldehyde dehydrogenase 1 expression); and (2) Their survival pathways (e.g., Wnt/β-catenin, Hedgehog, and Notch signaling pathway), in terms of expression of transcription factors/key proteins/microRNAs (miRNAs). Among the recently developed approaches to isolate CSCs, there are next generation sequencing (NGS) technologies. For example, Jonasson et al[24] isolated the stem-like subpopulation using a functional cellular assay that enriches for cells that can self-renew and differentiate, combined with NGS technologies (single-cell RNA sequencing) to identify CSCs[22-24]. Moreover, Rodriguez-Meira et al[25] in their scientific work developed an NGS platform that combines single-cell RNA-seq with mutational analysis allowing the identification of distinct subclones of cancer cells[26]. This evidence suggests that a combination of cell surface markers and functional assays provide an efficient tool for their identification.
CSCs’ properties have profound implications for treatment response due to their ability to evade conventional therapy and cause subsequent tumor relapse[27,28]. In this case, it is crucial to identify new treatment strategies that take into consideration the complexities of CSCs. Currently, global researchers are making great efforts to understand the biological properties of CSCs and develop new therapeutic approaches targeting CSCs.
EPIGENETIC MODULATORS
The term "epigenetics" is used to refer to information that controls gene expression that is stable and inheritable during cell division and happens without changes in the DNA sequence. Aberrant epigenetic landscapes control cell fate specification, promoting tumor initiation and progression[29,30].
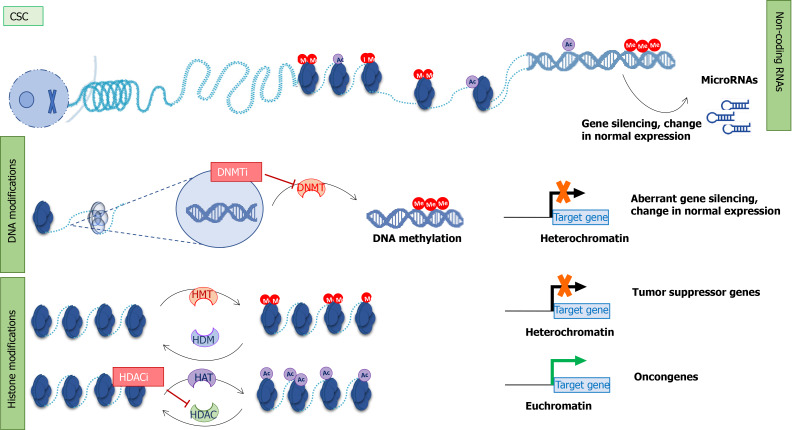
Epigenetics controls gene expression through three main mechanisms: DNA methylation, histone modifications, and non-coding RNA, particularly miRNAs[31] (Figure 1).
Figure 1.
Epigenetic mechanism in cancer stem cells and therapeutical approaches. Epigenetic modifiers: Non-coding RNA, DNA methylation, and histone modifications. Non-coding RNAs (microRNAs) regulate gene expression. microRNA expression can be regulated by other epigenetic modifiers. DNA methyltransferases add a methyl group to cytosine in the DNA sequence. This results in aberrant gene silencing and changes in normal gene expression. Posttranslational modifications of histone proteins (acetylation in purple and methylation in red) can affect chromatin structure. Histone enzymes add/remove acetyl or methyl groups. Histone deacetylases and DNMTs are the main targets of epigenetic inhibitors. CSC: Cancer stem cell; Me: Methyl group; Ac: Acetyl group; DNMT: DNA methyltransferases; HDAC: Histone deacetylases; HAT: Histone acetyltransferases; HMT: Histone methyltransferases; HDM: Histone demethylases; DNMTi: DNA methyltransferases inhibitors; HDACi: Histone deacetylases inhibitors.
DNA methylation is the most studied epigenetic modification. It is associated with transcriptional inactivation and closed chromatin structure, mainly regulating gene silencing or repression. It depends on the action of specialized enzymes, known as DNA methyltransferases (DNMTs) that transfer a methyl group from S-adenosyl methionine to the fifth carbon of the pyrimidine ring of a DNA base cytosine. DNA methylation occurs mainly at CpG dinucleotides, which are concentrated in specific regions of DNA called “CpG islands”. CpG islands are located at gene promoters, in regulatory regions, and in gene bodies[32], but DNA methylation could also be present in non-coding DNA sequences such as repetitive elements, transposons, non-coding RNAs, and introns[33,34].
A methylation pattern represents the fingerprint of a cell. It is established during early embryogenesis, and it is maintained during cell division by the action of DNMTs.
Methylation status is altered in cancers, mainly through two mechanisms: Regional hypermethylation and global hypomethylation[35]. DNA hypermethylation involves the CpG islands, which are usually unmethylated in normal cells. The result of DNA hypermethylation is the transcriptional repression of tumor-suppressor and tissue-specific genes, and the inactivation of miRNA, which are involved in the initiation and progression of cancer[34]. This mechanism occurs in different stages of carcinogenesis including CSC formation[36]. Conversely, global hypomethylation consists of the loss of the methyl group on cytosine, mainly in repetitive elements across DNA. Hypomethylation was one of the first epigenetic features discovered in human cancers[37], causing the reactivation of methylated regions of DNA such as transposons, introns, and germ-line genes that are silent in differentiated cells[34,38]. In high-grade cancers, such as glioblastoma (GBM), hypomethylation is a mechanism used by CSCs to reactivate key stem cell genes[39].
Abnormalities of DNA methylation are early events in pre-malignant transformation and are maintained in the global tumor population. However, the epigenome is in continuous evolution, and some of the changes are detectable in later steps of tumorigenesis as a result of positive selection. In this way, epigenome contributes to tumor heterogeneity and plasticity, which gives rise to a heterogeneous tumor composed of different cell subpopulations, one of them could have “stem-like” features. Additionally, compared to the bulk tumor, CSCs could acquire further epigenetic alterations in response to stress of different nature (e.g., chemotherapy/radiotherapy, chronic inflammation, and environmental exposures), contributing to tumor relapse[40].
The development of powerful “next-generation” techniques makes it possible to describe the entire epigenome of cells or tissues[38]. Capper et al[41] developed a DNA methylation-based classification for central nervous system cancers that allows discrimination between different subtypes of tumors, some of them previously considered as homogeneous diseases.
Histones are subject to reversible post-translational modifications (PTMs) that cooperate to govern the chromatin state. Histone PTMs influence chromatin structure that is conducive to the expression or repression of target genes. Histone amino-terminal regions can undergo diverse PTMs: Methylation, acetylation, phosphorylation, ubiquitylation, biotinylation, sumoylation, and ADP-ribosylation[42] that work in concert to define the chromatin status of the specific region of DNA.
Histone PTMs are controlled by different enzymes: "Writers" catalyze histone modifications; “erasers” cut histone modifications; and “readers” translate the PTMs’ language into cellular signals. All enzymes work together in a very specific manner to create the “histone code”.
Histone methylation and acetylation are the best-known PTMs on histone residues. Histone methylation is a reversible mechanism that occurs on lysine and arginine residues, leading to a different degree of methylation. Up to three methyl groups can be added to a single lysine residue (un-, mono-, di-, and tri-methylated states) and up to two groups to a single arginine residue (mono- and di-methylated states). Methylation can be either an activating or a repressive mark, depending on the target histone and lysine residue: For example, histone H3 Lysine 27 (H3K27) and histone H4 Lysine 20 (H4K20) are usually associated with gene silencing, while H3 Lysine 4 (H3K4) and H3 Lysine 36 (H3K36) are transcriptional activation marks. Histone acetylation is associated with relaxed chromatin structure and promotes the binding of transcription factors and RNA polymerase to DNA, resulting in the activation of gene expression[42].
Recent efforts have tried to highlight the role of epigenetic modulators in the development and plasticity of brain tumor CSCs[43,44]. In GBM CSCs (GSCs), Liau et al[45] showed in patient-derived GSCs, which effectively initiate tumors in mice models, that KDM6-mediated demethylation of histone H3 Lysine 27 trimethylation (H3K27me3), a repressive mark, has a central role in the maintenance and persistence of GSCs. Marampon et al[46] demonstrated the fundamental role of two histone deacetylases (HDAC4 and HDAC6) in regulating the DNA repair machinery, survival, and stemness characteristics of radioresistant GSCs derived from U87MG and U251MG human GMB cell lines subjected to irradiation. Moreover, a recent study by Banelli et al[47] on human GSCs derived from human tumors describes a subset of GSCs resistant to chemotherapy that are particularly dependent on KDM5A action, making them strongly sensitive to HDAC inhibitor (HDACi) treatments in term of cell viability, percentage of apoptotic cells, and reducing capacity of clonal growth. In medulloblastoma, pharmacological inhibition of histone methyltransferase enhancer of zeste 2 (EZH2) impairs proliferation and self-renewal of human and mouse stem-like cells, derived from primary human Sonic Hedgehog MB (SHH-MBs) and from tumors arisen in Ptc+/- mice, in in vitro and in vivo studies[48].
miRNAs and epigenetic modulators create a miRNA-epigenetic feedback loop: On the one hand, miRNA can regulate the transcription of epigenetics-associated enzymes, while on the other miRNA transcription is under the control of epigenetics machinery (DNA methylation, histone PTMs, and RNA modifications)[49].
In literature, there are several examples of miRNA-epigenetic machinery in CSCs. For example, Ferretti et al[50] described the role of the miR-326-ARRB1-E2F1 axis in medulloblastoma CSCs survival. For this study, MB CSCs were derived from tissues freshly resected from pediatric patients and cultured in CSC-enriched cultures. Notably, miR-326 is already described as onco-suppressor miRNA[50]. It is involved in neuronal differentiation, and its expression is under control of the histone-lysine N-methyltransferase EZH2. High levels of EZH2 are responsible for the presence of H3K27me3 on the promoter region of miR-326[22]. In another study, Lizarte Neto et al[51] investigated the role of miR-181d and the methylation status of the MGMT gene in response to pharmacological treatment in CD133+ GBM CSCs. Their results showed an increase of miR-181d and MGMT transcription levels after treatment, due to cellular mechanism of drug resistance[51].
The tumor microenvironment (TME) also acts as an epigenetic regulator for cancer cells. TME communicates with cancer cells through extracellular vesicles secreted by many TME cell types that contain various mediators including proteins and nucleic acids. Also, miRNAs can be charged in the extracellular vesicles and thereby alter the epigenome of the recipient cancer cell[52].
All this evidence makes brain CSCs an excellent candidate for pharmacological epigenetic treatments.
TRANSLATIONAL SIGNIFICANCE OF EPIGENETICS: EPIDRUGS
Epigenetics-based drugs (epidrugs) are chemical compounds that specifically target epigenetic marks (Figure 1). CSCs could acquire mutations in epigenetic marks or changing in methylation/acetylation status, or again in miRNAs signature, that make them sensitive to epigenetics-based drugs’ approaches. Epidrugs are based on the idea that by changing the epigenome of CSCs, it may be possible to remodel the fate of cells from CSCs to differentiated tumor cells[53-55]. Epidrugs are used as monotherapy or in combination with chemo-/radio-therapy to target both CSCs and bulk tumors. Today, several HDAC and DNMT inhibitors are in different clinical trial phases.
HDACi
Among the various epigenetic modulators, the acetylation state of histone proteins is one of the major targets for anticancer therapy. Histone acetylation is controlled by two types of enzymes: HDACs and histone acetyltransferases (Figure 1).
Human HDACs are categorized into four different classes: Class I (HDAC1, 2, 3, and 8), class IIa (HDAC4, 5, 7, and 9), class IIb (HDAC6 and 10), class III (sirt 1-7), and class IV (HDAC11). HDACs are enzymes that remove the acetyl group from lysine residues on histones, which compact the chromatin structure into a non-permissive state, resulting in transcriptional repression.
Based on isoform selectivity, HDACi can be classified as: (1) Pan-inhibitors, if they act against all HDACs; or (2) HDAC isoform-selective inhibitors if they target a specific HDAC class[56]. Pan-inhibitors are in turn classified according to their chemical structure as: (1) Hydroxamic acids; (2) Aliphatic carboxylic acids; (3) Benzamides; (4) Cyclic peptides; or (5) Sirtuin inhibitors.
HDACs control several cellular mechanisms. They have been implicated in different types of cancers[46], and several HDACs are overexpressed in brain cancers[57,58]. For example, Staberg et al[59] found an up-expression of HDAC1, 3, and 6 in 21 primary GBM cell cultures, grown as neurospheres, compared to non-neoplastic brain control cells (normal human astrocytes), and confirmed these findings in a panel of primary GBM tissue samples compared to normal brain tissues. They demonstrated the efficacy of HDACi therapy (trichostatin A) in GBM treatment in in vitro experiments.
HDACi exhibit anti-cancer activity against CSCs in tumors with a predominant stem-like population such as brain cancers. HDACi target the escape mechanism of CSCs, reversing chemo-radio-therapy resistance by inducing cell differentiation, apoptosis, inhibition of angiogenesis, and upregulation of tumor suppressor genes[60].
In line with this observation, da Cunha Jaeger et al[61] demonstrated that HDACi and mitogen-activated protein kinase/extracellular signal-regulated kinase inhibitors could modulate the stemness markers (BMI1 and CD133), viability, and neurosphere formation capacity of MB cell lines (DAOY and D283 cultured in serum-free sphere-induction medium). Their results proposed HDACi as a valid candidate for MB CSC treatment[61]. Coni et al[62] reported antitumor effects of selective HDAC1 and HDAC2 inhibition on SHH-MB cells and mouse models. Also, in diffuse intrinsic pontine glioma tumor models (patient-derived neurospheres, xenografts, and allografts), combination of HDAC and AXL inhibition modulate the H3K27M epigenetic mark resulting in a down-regulation of stemness markers (SOX2 and its target genes directly correlated with stem cell characteristics)[63].
Several HDACi are emerging as promising anticancer drugs in clinical trials for brain cancer treatment, both as combinatorial- and as mono-therapy[64]. Table 1 shows the HDACi used in clinical trials for brain cancers (Vorinostat, Valproic acid, Panobinostat, and Entinostat). Most clinical trials are focused on the usage of Vorinostat (suberoylanilide hydroxamic acid or SAHA), a potent inhibitor of HDAC classes I and II. Vorinostat was initially approved by the Food and Drug Administration for treating refractory cutaneous T-cell lymphoma, and its use was subsequently extended to various solid cancers[56]. In a phase 2 clinical trial, Vorinostat was tested as a mono-therapeutic agent in GBM treatment to target both CSCs and differentiated cancer cells. In fact, preclinical studies demonstrated the efficacy of Vorinostat in reducing EZH2 and CD133 stemness marker in patient-derived GBM CSCs[65] and in modulating senescence via the p38-p53 axis and inducing apoptosis in GBM cell lines cultured in serum-free sphere-induction medium[66]. Sung et al[67] demonstrated that a combination of Vorinostat with melatonin can overcome the pharmacological resistance of human GBM CSCs, reducing self-renewal and proliferation of stem cell compartments and increasing apoptosis markers (cleaved poly-ADP ribose polymerase and p-γH2AX).
Table 1.
Histone deacetylases inhibitors in clinical trial for brain cancer treatment
|
Compound
|
Class
|
Target
|
Conditions
|
Phase of clinical trial
|
Study title
|
| Entinostat | iHDAC (benzamide derivates) | Class I | Brain stem neoplasm | Phase I | Entinostat in Treating Pediatric Patients With Recurrent or Refractory Solid Tumors |
| Pineal region neoplasm | |||||
| Recurrent lymphoma | |||||
| Recurrent malignant solid neoplasm | |||||
| Recurrent primary central nervous system neoplasm | |||||
| Recurrent visual pathway glioma | |||||
| Refractory lymphoma | |||||
| Refractory malignant solid neoplasm | |||||
| Refractory primary central nervous system neoplasm | |||||
| Refractory visual pathway glioma | |||||
| Panobinostat (LBH589) | iHDAC (hydroxymic acids) | Class I, II, IV | Recurrent glioma | Phase I | Panobinostat and Stereotactic Radiation Therapy in Treating Patients With Brain Tumors |
| High-grade meningioma | |||||
| Brain metastasis | |||||
| Panobinostat (LBH589) | iHDAC (hydroxymic acids) | Class I, II, IV | Diffuse intrinsic pontine glioma | Phase I | Phase I Study of Marizomib + Panobinostat for Children With DIPG |
| Pediatric brainstem glioma | |||||
| Pediatric brainstem gliosarcoma recurrent pediatric cancer | |||||
| Pediatric brain tumor | |||||
| Diffuse glioma | |||||
| Panobinostat (LBH589) | iHDAC (hydroxymic acids) | Class I, II, IV | Recurrent malignant gliomas | Phase II | Phase II Trial of LBH589 (Panobinostat) in Adult Patients With Recurrent Malignant Gliomas |
| Valproic Acid | iHDAC (fatty acid derivates) | Class I, II | GBM WHO grade IV | Phase III | International Cooperative Phase III Trial of the HIT-HGG Study Group (HIT-HGG-2013) |
| Diffuse midline glioma histone 3 K27M WHO grade IV | |||||
| Anaplastic astrocytoma WHO grade III | |||||
| Diffuse intrinsic pontine glioma | |||||
| Gliomatosis cerebri | |||||
| Valproic Acid | iHDAC (fatty acid derivates) | Class I, II | Brain metastasis | Phase I | Phase I Study of Temozolomide, Valproic Acid and Radiation Therapy in Patients With Brain Metastases |
| Valproic Acid | iHDAC (fatty acid derivates) | Class I, II | Neuroectodermal tumor | Phase I | Valproate and Etoposide for Patients With Neuronal Tumors and Brain Metastases |
| Brain metastases | |||||
| Advanced cancer | |||||
| Valproic Acid | iHDAC (fatty acid derivates) | Class I, II | Brain tumors | Phase II | Valproic Acid With Temozolomide and Radiation Therapy to Treat Brain Tumors |
| High grade gliomas | |||||
| Vorinostat (SAHA) | iHDAC (hydroxymic acids) | Pan-HDAC | Brain cancer | Phase I/II | Phase I/II Vorinostat, Erlotinib and Temozolomide for Recurrent Glioblastoma Multiforme |
| GBM multiforme | |||||
| Vorinostat (SAHA) | iHDAC (hydroxymic acids) | Pan-HDAC | Adult giant cell GBM | Phase II | Vorinostat and Bortezomib in Treating Patients With Progressive, Recurrent Glioblastoma Multiforme |
| Adult GBM | |||||
| Adult gliosarcoma | |||||
| Recurrent adult brain tumor | |||||
| Vorinostat (SAHA) | iHDAC (hydroxymic acids) | Pan-HDAC | Adult giant cell GBM | Phase II | Vorinostat in Treating Patients With Progressive or Recurrent Glioblastoma Multiforme |
| Adult GBM | |||||
| Adult gliosarcoma | |||||
| Recurrent adult brain tumor | |||||
| Vorinostat (SAHA) | iHDAC (hydroxymic acids) | Pan-HDAC | Recurrent GBM multiforme | Phase II | Ph II SAHA and Bevacizumab for Recurrent Malignant Glioma Patients |
| Malignant glioma | |||||
| Adult brain tumor | |||||
| Vorinostat (SAHA) | iHDAC (hydroxymic acids) | Pan-HDAC | Brain cancer | Phase I | Phase I Vorinostat Concurrent With Stereotactic Radiosurgery (SRS) in Brain Metastases From Non-Small Cell Lung Cancer |
| Neoplasm metastasis | |||||
| Lung cancer | |||||
| Carcinoma, non-small-cell lung | |||||
| Vorinostat (SAHA) | iHDAC (hydroxymic acids) | Pan-HDAC | Brain tumor | Phase I/II | Suberoylanilide Hydroxamic Acid (SAHA), Bevacizumab, Daily Temozolomide for Recurrent Malignant Gliomas |
| GBM | |||||
| Vorinostat (SAHA) | iHDAC (hydroxymic acids) | Pan-HDAC | Brain metastases | Phase I | Study of the Combination of Vorinostat and Radiation Therapy for the Treatment of Patients With Brain Metastases |
| Vorinostat (SAHA) | iHDAC (hydroxymic acids) | Pan-HDAC | Adult GBM | Not applicable | Magnetic Resonance Spectroscopy Imaging in Predicting Response to Vorinostat and Temozolomide in Patients With Recurrent or Progressive Glioblastoma |
| Depression | |||||
| Recurrent adult brain tumor | |||||
| Vorinostat (SAHA) | iHDAC (hydroxymic acids) | Pan-HDAC | Adult anaplastic astrocytoma | Phase I | Vorinostat and Temozolomide in Treating Patients With Malignant Gliomas |
| Adult anaplastic oligodendrogliomaAdult giant cell GBM | |||||
| Adult GBM | |||||
| Adult gliosarcomaAdult mixed glioma | |||||
| Recurrent adult brain neoplasm | |||||
| Vorinostat (SAHA) | iHDAC (hydroxymic acids) | Pan-HDAC | Medulloblastoma | Phase I | Vorinostat Combined With Isotretinoin and Chemotherapy in Treating Younger Patients With Embryonal Tumors of the Central Nervous System |
| Pineoblastoma | |||||
| Supratentorial embryonal tumor | |||||
| Vorinostat (SAHA) | iHDAC (hydroxymic acids) | Pan-HDAC | Brain metastasis | Phase II | Vorinostat and Concurrent Whole Brain Radiotherapy for Brain Metastasis |
| Vorinostat (SAHA) | iHDAC (hydroxymic acids) | Pan-HDAC | Adult anaplastic astrocytoma | Phase I | High-Dose Vorinostat and Fractionated Stereotactic Body Radiation Therapy in Treating Patients With Recurrent Glioma |
| Adult anaplastic oligodendroglioma | |||||
| Adult giant cell GBM | |||||
| Adult GBM | |||||
| Adult gliosarcoma | |||||
| Adult mixed glioma | |||||
| Recurrent adult brain neoplasm | |||||
| Vorinostat (SAHA) | iHDAC (hydroxymic acids) | Pan-HDAC | GBM | Phase I | Pembrolizumab and Vorinostat Combined With Temozolomide for Newly Diagnosed Glioblastoma |
| Brain tumor | |||||
| GBM | |||||
| Vorinostat (SAHA) | iHDAC (hydroxymic acids) | Pan-HDAC | Brain stem glioma | Phase II/III | Vorinostat, Temozolomide, or Bevacizumab in Combination With Radiation Therapy Followed by Bevacizumab and Temozolomide in Young Patients With Newly Diagnosed High-Grade Glioma |
| Cerebral astrocytoma | |||||
| Childhood cerebellar anaplastic astrocytoma | |||||
| Childhood cerebral anaplastic astrocytoma | |||||
| Childhood spinal cord neoplasm | |||||
| Vorinostat (SAHA) | iHDAC (hydroxymic acids) | Pan-HDAC | Adult GBM | Not applicable | Magnetic Resonance Spectroscopy Imaging in Predicting Response to Vorinostat and Temozolomide in Patients With Recurrent or Progressive Glioblastoma |
| Depression | |||||
| Recurrent adult brain tumor | |||||
| Vorinostat (SAHA) | iHDAC (hydroxymic acids) | Pan-HDAC | Adult anaplastic astrocytoma | Phase I | Vorinostat and Temozolomide in Treating Patients With Malignant Gliomas |
| Adult anaplastic oligodendroglioma | |||||
| Adult giant cell GBM | |||||
| Adult GBM | |||||
| Adult gliosarcoma | |||||
| Adult mixed glioma | |||||
| Recurrent adult brain neoplasm | |||||
| Vorinostat (SAHA) | iHDAC (hydroxymic acids) | Pan-HDAC | Medulloblastoma | Phase I | Vorinostat Combined With Isotretinoin and Chemotherapy in Treating Younger Patients With Embryonal Tumors of the Central Nervous System |
| Pineoblastoma | |||||
| Supratentorial embryonal tumor | |||||
| Vorinostat (SAHA) | iHDAC (hydroxymic acids) | Pan-HDAC | Brain metastasis | Phase II | Vorinostat and Concurrent Whole Brain Radiotherapy for Brain Metastasis |
| Vorinostat (SAHA) | iHDAC (hydroxymic acids) | Pan-HDAC | Adult anaplastic astrocytoma | Phase I | High-Dose Vorinostat and Fractionated Stereotactic Body Radiation Therapy in Treating Patients With Recurrent Glioma |
| Adult anaplastic oligodendroglioma | |||||
| Adult giant cell GBM | |||||
| Adult GBM | |||||
| Adult gliosarcoma | |||||
| Adult mixed glioma | |||||
| Recurrent adult brain neoplasm | |||||
| Vorinostat (SAHA) | iHDAC (hydroxymic acids) | Pan-HDAC | GBM | Phase I | Pembrolizumab and Vorinostat Combined With Temozolomide for Newly Diagnosed GBM |
| Brain tumor | |||||
| GBM | |||||
| Vorinostat (SAHA) | iHDAC (hydroxymic acids) | Pan-HDAC | Brain stem glioma | Phase II/III | Vorinostat, Temozolomide, or Bevacizumab in Combination With Radiation Therapy Followed by Bevacizumab and Temozolomide in Young Patients With Newly Diagnosed High-Grade Glioma |
| Cerebral astrocytoma | |||||
| Childhood cerebellar anaplastic astrocytoma | |||||
| Childhood cerebral anaplastic astrocytoma | |||||
| Childhood spinal cord neoplasm | |||||
| Vorinostat (SAHA) | iHDAC (hydroxymic acids) | Pan-HDAC | Childhood atypical teratoid/rhabdoid tumor | Phase I | Vorinostat and Temozolomide in Treating Young Patients With Relapsed or Refractory Primary Brain Tumors or Spinal Cord Tumors |
| Childhood central nervous system choriocarcinoma | |||||
| Childhood central nervous system embryonal tumor and other |
HDAC: Histone deacetylases; iHDAC: HDAC inhibitors; DIPG: Diffuse intrinsic pontine gliomas; WHO: World Health Organization; Ph: Phase; SRS: Stereotactic radiosurgery; GBM: Glioblastoma.
Vorinostat acts by inducing the acetylation of proteins, including histones and transcription factors at both transcriptional and non-transcriptional levels, leading to different cellular effects[68]. Vorinostat, like other pan-HDACi, has variable activity against HDAC isoenzymes, some of which are important in antitumor response.
Vorinostat shows a different toxicity profile compared to classical chemotherapeutic drugs. In fact, common pan-HDACi side effects include fatigue, nausea/vomiting, anemia, anorexia, increased blood urea, hyperglycemia, and thrombocytopenia. Some of these adverse effects can be routinely managed by physicians, but some others need more careful monitoring[69]. However, side effects are dose-dependent and Vorinostat is effective at very low concentrations[70]. There are several reasons behind the epi-drugs’ toxicity. It is partly due to “on-target” effects, which could be explained with the concept of “pleiotropy”. Specifically, a single target gene could be involved in different signaling and controls multiple phenotypic effects. Another reason is “off-target” effects. Epi-drugs are designed to inhibit aberrant epigenetic enzymes, but it is known that they could also affect other classes of substrates belonging to unintended cellular pathways, at both intracellular and extracellular levels[71].
In medulloblastoma, the administration of epigenetic modifiers such as Vorinostat and Valproic acid shows radiosynergistic action on the proliferation and cloning capacity of three human MB cell lines (DAOY, MEB-Med8a, and D283-Med), offering a new opportunity to treat MB patients[72].
DNA methyltransferase inhibitors
The readout of DNMTs is hypermethylation of the DNA sequence and resulting silencing of gene expression. Aberrant DNA methylation has been associated with different stages of cancers. This evidence supports a rationale for using DNMT inhibitors (DNMTi) in cancer treatment (Figure 1).
There are two different classes of DNMTi: (1) Nucleoside analogues, which could be considered analogues to cytosine and act as a natural substrate for DNMT (e.g., 5-azacytidine); and (2) Nonnucleoside compounds, which inhibit DNA methyltransferase activity through mechanisms other than DNA incorporation.
This second class of DNMTi appears to be less toxic and more stable than nucleoside analogues[5,73,74], and Valente and colleagues have focused on the study of nonnucleoside compounds[75].
The archetypal DNA methyltransferase inhibitor is 5-azacytidine (also known as 5-aza), it is a nucleoside inhibitor that is incorporated into the DNA sequence and covalently bonds to and inactivates DNA methyltransferase. As shown in Table 2, 5-azacytidine has been tested in different phase I trials against various brain cancers, particularly in recurrent brain tumors, GBM, and ependymoma.
Table 2.
DNA methyltransferase inhibitors in clinical trial for brain cancer treatment
|
Compound
|
Class
|
Target
|
Conditions
|
Phase of clinical trial
|
Study title
|
| 5-Azacytidine | DNMTi | Pan-DNMT | Brain tumor recurrent | Early Phase 1 | Infusion of 5-Azacytidine (5-AZA) Into the Fourth Ventricle in Children With Recurrent Posterior Fossa Ependymoma |
| Azacitidine | DNMTi | Pan-DNMT | GBM multiformeOther | Phase 1 | Bioequivalence & Food Effect Study in Patients With Solid Tumor or Hematologic Malignancies |
| 5-Azacytidine | DNMTi | Pan-DNMT | Recurrent childhood CNS tumor | Phase 1 | Treatment of Children With Recurrent Refractory Brain/Solid Tumors and Recurrent Ependymoma |
| Ependymoma, recurrent childhood | |||||
| Childhood solid tumor |
DNMT: DNA methyltransferase; DNMTi: DNMT inhibitors; CNS: Central nervous system; GBM: Glioblastoma.
Several studies have also demonstrated the differentiation potential of DNMTi in cancer therapy. Liao et al[76] showed that decitabine, an analogue of cytosine, in combinatorial treatment with a differentiation drug, could provide an effective route to enhancing cell differentiation (oligodendrocyte-like morphology and mRNA expression of terminal differentiation marker MBP) and inhibiting cell growth in two malignant glioma human cell lines. Andrade et al[77] tested the efficacy of zebularine, another DNMTi, in four pediatric SHH-MB cell lines (DAOY, ONS-76, UW402, and UW473). Zebularine decreased MB cell growth by targeting the Sonic Hedgehog pathway’s components (GLI1, SMO and PTCH1), evaluated at transcriptional levels. This provides a rationale for further in vivo investigation into the combination of zebularine with chemotherapy[77]. Valente et al[75] tested two nonnucleosides DNMTi (compounds 2 and 5) in mouse MB stem cells isolated from fresh tumor specimens from Ptch1+/- mouse models; compound 2 significantly blocked cell proliferation, while compound 5 was stronger in differentiation potential evaluated by both βIII-tubulin reverse transcriptase polymerase chain reaction and morphology images.
CONCLUSION
Aberrant epigenetic regulation has emerged as a key player in the genesis and progression of brain tumors, influencing malignant phenotypes at various stages of the disease and possibly underlying individual variability in drug response. In particular, the epigenome of brain tumors can be modulated by both cell-intrinsic (e.g., mutations) and cell-extrinsic (e.g., microenvironment) mechanisms, favoring those characteristics of CSCs responsible for cancer growth and disease progression. Reprogramming the epigenetic landscape in the cancer epigenome is among the most promising target therapies, both as a treatment itself and for reversing drug resistance. In this review, we discussed how epigenetic alterations regulate the "stem-like" properties of CSCs and the epigenetic drugs available to blockade epi-mutations. We have reported several examples of epidrugs in Phase I/II clinical trials, providing evidence on the benefit of using epidrugs as single agents or in combination-therapy in brain tumors. We believe this is thus a viable avenue for clinical trials aiming at the development of more affordable and efficient anticancer drugs and treatments.
ACKNOWLEDGEMENTS
We thank Ralf Mouthaan for manuscript editing.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Manuscript source: Invited manuscript
Peer-review started: March 13, 2021
First decision: April 6, 2021
Article in press: June 22, 2021
Specialty type: Oncology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Velasco-Velazquez M S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Xing YX
Contributor Information
Luana Abballe, Department of Pediatric Hematology/Oncology and Cellular and Gene Therapy, Bambino Gesù Children's Hospital, IRCCS, Rome 00165, Italy.
Evelina Miele, Department of Pediatric Hematology/Oncology and Cellular and Gene Therapy, Bambino Gesù Children's Hospital, IRCCS, Rome 00165, Italy. evelina.miele@opbg.net.
References
- 1.Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds) SEER Cancer Statistics Review 1975-2017. National Cancer Institute. Bethesda, MD, based on November 2019 SEER data submission, posted to the SEER web site, April 2020. Available from: https://seer.cancer.gov/csr/1975_2017/
- 2.Pollack IF, Agnihotri S, Broniscer A. Childhood brain tumors: current management, biological insights, and future directions. J Neurosurg Pediatr . 2019;23:261–273. doi: 10.3171/2018.10.PEDS18377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mackay A, Burford A, Carvalho D, Izquierdo E, Fazal-Salom J, Taylor KR, Bjerke L, Clarke M, Vinci M, Nandhabalan M, Temelso S, Popov S, Molinari V, Raman P, Waanders AJ, Han HJ, Gupta S, Marshall L, Zacharoulis S, Vaidya S, Mandeville HC, Bridges LR, Martin AJ, Al-Sarraj S, Chandler C, Ng HK, Li X, Mu K, Trabelsi S, Brahim DH, Kisljakov AN, Konovalov DM, Moore AS, Carcaboso AM, Sunol M, de Torres C, Cruz O, Mora J, Shats LI, Stavale JN, Bidinotto LT, Reis RM, Entz-Werle N, Farrell M, Cryan J, Crimmins D, Caird J, Pears J, Monje M, Debily MA, Castel D, Grill J, Hawkins C, Nikbakht H, Jabado N, Baker SJ, Pfister SM, Jones DTW, Fouladi M, von Bueren AO, Baudis M, Resnick A, Jones C. Integrated Molecular Meta-Analysis of 1,000 Pediatric High-Grade and Diffuse Intrinsic Pontine Glioma. Cancer Cell . 2017;32:520–537.e5. doi: 10.1016/j.ccell.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magee JA, Piskounova E, Morrison SJ. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell . 2012;21:283–296. doi: 10.1016/j.ccr.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghasemi S. Cancer's epigenetic drugs: where are they in the cancer medicines? Pharmacogenomics J . 2020;20:367–379. doi: 10.1038/s41397-019-0138-5. [DOI] [PubMed] [Google Scholar]
- 6.Baumann M, Krause M, Hill R. Clonogens and cancer stem cells. Nat Rev Cancer . 2001;8:990. doi: 10.1038/nrc2419. [DOI] [PubMed] [Google Scholar]
- 7.Silver DJ, Lathia JD. Revealing the glioma cancer stem cell interactome, one niche at a time. J Pathol . 2018;244:260–264. doi: 10.1002/path.5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med . 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 9.Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia . 2002;39:193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- 10.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res . 2003;63:5821–5828. [PubMed] [Google Scholar]
- 11.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature . 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 12.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res . 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 13.Taylor MD, Poppleton H, Fuller C, Su X, Liu Y, Jensen P, Magdaleno S, Dalton J, Calabrese C, Board J, Macdonald T, Rutka J, Guha A, Gajjar A, Curran T, Gilbertson RJ. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell . 2005;8:323–335. doi: 10.1016/j.ccr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Po A, Ferretti E, Miele E, De Smaele E, Paganelli A, Canettieri G, Coni S, Di Marcotullio L, Biffoni M, Massimi L, Di Rocco C, Screpanti I, Gulino A. Hedgehog controls neural stem cells through p53-independent regulation of Nanog. EMBO J . 2010;29:2646–2658. doi: 10.1038/emboj.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manoranjan B, Wang X, Hallett RM, Venugopal C, Mack SC, McFarlane N, Nolte SM, Scheinemann K, Gunnarsson T, Hassell JA, Taylor MD, Lee C, Triscott J, Foster CM, Dunham C, Hawkins C, Dunn SE, Singh SK. FoxG1 interacts with Bmi1 to regulate self-renewal and tumorigenicity of medulloblastoma stem cells. Stem Cells . 2013;31:1266–1277. doi: 10.1002/stem.1401. [DOI] [PubMed] [Google Scholar]
- 16.Tirosh I, Venteicher AS, Hebert C, Escalante LE, Patel AP, Yizhak K, Fisher JM, Rodman C, Mount C, Filbin MG, Neftel C, Desai N, Nyman J, Izar B, Luo CC, Francis JM, Patel AA, Onozato ML, Riggi N, Livak KJ, Gennert D, Satija R, Nahed BV, Curry WT, Martuza RL, Mylvaganam R, Iafrate AJ, Frosch MP, Golub TR, Rivera MN, Getz G, Rozenblatt-Rosen O, Cahill DP, Monje M, Bernstein BE, Louis DN, Regev A, Suvà ML. Single-cell RNA-seq supports a developmental hierarchy in human oligodendroglioma. Nature . 2016;539:309–313. doi: 10.1038/nature20123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim WT, Ryu CJ. Cancer stem cell surface markers on normal stem cells. BMB Rep . 2017;50:285–298. doi: 10.5483/BMBRep.2017.50.6.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U, Beier CP. CD133(+) and CD133(-) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res . 2007;67:4010–4015. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- 19.Singh SK, Venugopal C. Brain Tumor Stem Cells Methods and Protocols Methods in Molecular Biology 1869. Humana Press, New York: Springer New York, 2019: 1869. [Google Scholar]
- 20.Glumac PM, LeBeau AM. The role of CD133 in cancer: a concise review. Clin Transl Med . 2018;7:18. doi: 10.1186/s40169-018-0198-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng JX, Liu BL, Zhang X. How powerful is CD133 as a cancer stem cell marker in brain tumors? Cancer Treat Rev . 2009;35:403–408. doi: 10.1016/j.ctrv.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Miele E, Po A, Mastronuzzi A, Carai A, Besharat ZM, Pediconi N, Abballe L, Catanzaro G, Sabato C, De Smaele E, Canettieri G, Di Marcotullio L, Vacca A, Mai A, Levrero M, Pfister SM, Kool M, Giangaspero F, Locatelli F, Ferretti E. Downregulation of miR-326 and its host gene β-arrestin1 induces pro-survival activity of E2F1 and promotes medulloblastoma growth. Mol Oncol . 2021;15:523–542. doi: 10.1002/1878-0261.12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abbaszadegan MR, Bagheri V, Razavi MS, Momtazi AA, Sahebkar A, Gholamin M. Isolation, identification, and characterization of cancer stem cells: A review. J Cell Physiol . 2017;232:2008–2018. doi: 10.1002/jcp.25759. [DOI] [PubMed] [Google Scholar]
- 24.Jonasson E, Ghannoum S, Persson E, Karlsson J, Kroneis T, Larsson E, Landberg G, Ståhlberg A. Identification of Breast Cancer Stem Cell Related Genes Using Functional Cellular Assays Combined With Single-Cell RNA Sequencing in MDA-MB-231 Cells. Front Genet . 2019;10:500. doi: 10.3389/fgene.2019.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez-Meira A, Buck G, Clark SA, Povinelli BJ, Alcolea V, Louka E, McGowan S, Hamblin A, Sousos N, Barkas N, Giustacchini A, Psaila B, Jacobsen SEW, Thongjuea S, Mead AJ. Unravelling Intratumoral Heterogeneity through High-Sensitivity Single-Cell Mutational Analysis and Parallel RNA Sequencing. Mol Cell. 2019;73:1292–1305.e8. doi: 10.1016/j.molcel.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akbarzadeh M, Maroufi NF, Tazehkand AP, Akbarzadeh M, Bastani S, Safdari R, Farzane A, Fattahi A, Nejabati HR, Nouri M, Samadi N. Current approaches in identification and isolation of cancer stem cells. J Cell Physiol . 2019 doi: 10.1002/jcp.28271. [DOI] [PubMed] [Google Scholar]
- 27.Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, Parada LF. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature . 2012;488:522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature . 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 29.Elsässer SJ, Allis CD, Lewis PW. Cancer. New epigenetic drivers of cancers. Science . 2011;331:1145–1146. doi: 10.1126/science.1203280. [DOI] [PubMed] [Google Scholar]
- 30.Northcott PA, Lee C, Zichner T, Stütz AM, Erkek S, Kawauchi D, Shih DJ, Hovestadt V, Zapatka M, Sturm D, Jones DT, Kool M, Remke M, Cavalli FM, Zuyderduyn S, Bader GD, VandenBerg S, Esparza LA, Ryzhova M, Wang W, Wittmann A, Stark S, Sieber L, Seker-Cin H, Linke L, Kratochwil F, Jäger N, Buchhalter I, Imbusch CD, Zipprich G, Raeder B, Schmidt S, Diessl N, Wolf S, Wiemann S, Brors B, Lawerenz C, Eils J, Warnatz HJ, Risch T, Yaspo ML, Weber UD, Bartholomae CC, von Kalle C, Turányi E, Hauser P, Sanden E, Darabi A, Siesjö P, Sterba J, Zitterbart K, Sumerauer D, van Sluis P, Versteeg R, Volckmann R, Koster J, Schuhmann MU, Ebinger M, Grimes HL, Robinson GW, Gajjar A, Mynarek M, von Hoff K, Rutkowski S, Pietsch T, Scheurlen W, Felsberg J, Reifenberger G, Kulozik AE, von Deimling A, Witt O, Eils R, Gilbertson RJ, Korshunov A, Taylor MD, Lichter P, Korbel JO, Wechsler-Reya RJ, Pfister SM. Enhancer hijacking activates GFI1 family oncogenes in medulloblastoma. Nature . 2014;511:428–434. doi: 10.1038/nature13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feinberg AP, Tycko B. The history of cancer epigenetics. Nat Rev Cancer . 2004;4:143–153. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- 32.Alelú-Paz R, Ashour N, González-Corpas A, Ropero S. DNA methylation, histone modifications, and signal transduction pathways: a close relationship in malignant gliomas pathophysiology. J Signal Transduct . 2012;2012:956958. doi: 10.1155/2012/956958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lujambio A, Portela A, Liz J, Melo SA, Rossi S, Spizzo R, Croce CM, Calin GA, Esteller M. CpG island hypermethylation-associated silencing of non-coding RNAs transcribed from ultraconserved regions in human cancer. Oncogene . 2010;29:6390–6401. doi: 10.1038/onc.2010.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ellis L, Atadja PW, Johnstone RW. Epigenetics in cancer: targeting chromatin modifications. Mol Cancer Ther . 2009;8:1409–1420. doi: 10.1158/1535-7163.MCT-08-0860. [DOI] [PubMed] [Google Scholar]
- 35.Takeshima H, Ushijima T. DNA methylation changes in cancer: Mechanisms. In: Boffetta P, Hainaut P. Encyclopedia of Cancer. 3rd ed. Elsevier, 2018: 520-529. [Google Scholar]
- 36.Ohm JE, McGarvey KM, Yu X, Cheng L, Schuebel KE, Cope L, Mohammad HP, Chen W, Daniel VC, Yu W, Berman DM, Jenuwein T, Pruitt K, Sharkis SJ, Watkins DN, Herman JG, Baylin SB. A stem cell-like chromatin pattern may predispose tumor suppressor genes to DNA hypermethylation and heritable silencing. Nat Genet . 2007;39:237–242. doi: 10.1038/ng1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feinberg AP, Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature . 1983;301:89–92. doi: 10.1038/301089a0. [DOI] [PubMed] [Google Scholar]
- 38.Toraño EG, Petrus S, Fernandez AF, Fraga MF. Global DNA hypomethylation in cancer: review of validated methods and clinical significance. Clin Chem Lab Med . 2012;50:1733–1742. doi: 10.1515/cclm-2011-0902. [DOI] [PubMed] [Google Scholar]
- 39.Gopisetty G, Xu J, Sampath D, Colman H, Puduvalli VK. Epigenetic regulation of CD133/PROM1 expression in glioma stem cells by Sp1/myc and promoter methylation. Oncogene . 2013;32:3119–3129. doi: 10.1038/onc.2012.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Easwaran H, Tsai HC, Baylin SB. Cancer epigenetics: tumor heterogeneity, plasticity of stem-like states, and drug resistance. Mol Cell . 2014;54:716–727. doi: 10.1016/j.molcel.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Capper D, Jones DTW, Sill M, Hovestadt V, Schrimpf D, Sturm D, Koelsche C, Sahm F, Chavez L, Reuss DE, Kratz A, Wefers AK, Huang K, Pajtler KW, Schweizer L, Stichel D, Olar A, Engel NW, Lindenberg K, Harter PN, Braczynski AK, Plate KH, Dohmen H, Garvalov BK, Coras R, Hölsken A, Hewer E, Bewerunge-Hudler M, Schick M, Fischer R, Beschorner R, Schittenhelm J, Staszewski O, Wani K, Varlet P, Pages M, Temming P, Lohmann D, Selt F, Witt H, Milde T, Witt O, Aronica E, Giangaspero F, Rushing E, Scheurlen W, Geisenberger C, Rodriguez FJ, Becker A, Preusser M, Haberler C, Bjerkvig R, Cryan J, Farrell M, Deckert M, Hench J, Frank S, Serrano J, Kannan K, Tsirigos A, Brück W, Hofer S, Brehmer S, Seiz-Rosenhagen M, Hänggi D, Hans V, Rozsnoki S, Hansford JR, Kohlhof P, Kristensen BW, Lechner M, Lopes B, Mawrin C, Ketter R, Kulozik A, Khatib Z, Heppner F, Koch A, Jouvet A, Keohane C, Mühleisen H, Mueller W, Pohl U, Prinz M, Benner A, Zapatka M, Gottardo NG, Driever PH, Kramm CM, Müller HL, Rutkowski S, von Hoff K, Frühwald MC, Gnekow A, Fleischhack G, Tippelt S, Calaminus G, Monoranu CM, Perry A, Jones C, Jacques TS, Radlwimmer B, Gessi M, Pietsch T, Schramm J, Schackert G, Westphal M, Reifenberger G, Wesseling P, Weller M, Collins VP, Blümcke I, Bendszus M, Debus J, Huang A, Jabado N, Northcott PA, Paulus W, Gajjar A, Robinson GW, Taylor MD, Jaunmuktane Z, Ryzhova M, Platten M, Unterberg A, Wick W, Karajannis MA, Mittelbronn M, Acker T, Hartmann C, Aldape K, Schüller U, Buslei R, Lichter P, Kool M, Herold-Mende C, Ellison DW, Hasselblatt M, Snuderl M, Brandner S, Korshunov A, von Deimling A, Pfister SM. DNA methylation-based classification of central nervous system tumours. Nature . 2018;555:469–474. doi: 10.1038/nature26000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hosseini A, Minucci S. Alterations of Histone Modifications in Cancer. In: Tollefsbol TO. In Translational Epigenetics, Epigenetics in Human Disease. 2nd ed. Elsevier Inc, 2018: 141-217. [Google Scholar]
- 43.Zhou D, Alver BM, Li S, Hlady RA, Thompson JJ, Schroeder MA, Lee JH, Qiu J, Schwartz PH, Sarkaria JN, Robertson KD. Distinctive epigenomes characterize glioma stem cells and their response to differentiation cues. Genome Biol . 2018;19:43. doi: 10.1186/s13059-018-1420-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCann TS, Sobral LM, Self C, Hsieh J, Sechler M, Jedlicka P. Biology and targeting of the Jumonji-domain histone demethylase family in childhood neoplasia: a preclinical overview. Expert Opin Ther Targets . 2019;23:267–280. doi: 10.1080/14728222.2019.1580692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liau BB, Sievers C, Donohue LK, Gillespie SM, Flavahan WA, Miller TE, Venteicher AS, Hebert CH, Carey CD, Rodig SJ, Shareef SJ, Najm FJ, van Galen P, Wakimoto H, Cahill DP, Rich JN, Aster JC, Suvà ML, Patel AP, Bernstein BE. Adaptive Chromatin Remodeling Drives Glioblastoma Stem Cell Plasticity and Drug Tolerance. Cell Stem Cell . 2017;20:233–246.e7. doi: 10.1016/j.stem.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marampon F, Megiorni F, Camero S, Crescioli C, McDowell HP, Sferra R, Vetuschi A, Pompili S, Ventura L, De Felice F, Tombolini V, Dominici C, Maggio R, Festuccia C, Gravina GL. HDAC4 and HDAC6 sustain DNA double strand break repair and stem-like phenotype by promoting radioresistance in glioblastoma cells. Cancer Lett . 2017;397:1–11. doi: 10.1016/j.canlet.2017.03.028. [DOI] [PubMed] [Google Scholar]
- 47.Banelli B, Carra E, Barbieri F, Würth R, Parodi F, Pattarozzi A, Carosio R, Forlani A, Allemanni G, Marubbi D, Florio T, Daga A, Romani M. The histone demethylase KDM5A is a key factor for the resistance to temozolomide in glioblastoma. Cell Cycle . 2015;14:3418–3429. doi: 10.1080/15384101.2015.1090063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miele E, Valente S, Alfano V, Silvano M, Mellini P, Borovika D, Marrocco B, Po A, Besharat ZM, Catanzaro G, Battaglia G, Abballe L, Zwergel C, Stazi G, Milite C, Castellano S, Tafani M, Trapencieris P, Mai A, Ferretti E. The histone methyltransferase EZH2 as a druggable target in SHH medulloblastoma cancer stem cells. Oncotarget . 2017;8:68557–68570. doi: 10.18632/oncotarget.19782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao Q, Chen Y, Zhou X. The roles of microRNAs in epigenetic regulation. Curr Opin Chem Biol . 2019;51:11–17. doi: 10.1016/j.cbpa.2019.01.024. [DOI] [PubMed] [Google Scholar]
- 50.Ferretti E, De Smaele E, Miele E, Laneve P, Po A, Pelloni M, Paganelli A, Di Marcotullio L, Caffarelli E, Screpanti I, Bozzoni I, Gulino A. Concerted microRNA control of Hedgehog signalling in cerebellar neuronal progenitor and tumour cells. EMBO J . 2008;27:2616–2627. doi: 10.1038/emboj.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lizarte Neto FS, Rodrigues AR, Trevisan FA, de Assis Cirino ML, Matias CCMS, Pereira-da-Silva G, Peria FM, Tirapelli DPDC, Carlotti CG Jr. microRNA-181d associated with the methylation status of the MGMT gene in Glioblastoma multiforme cancer stem cells submitted to treatments with ionizing radiation and temozolomide. Brain Res . 2019;1720:146302. doi: 10.1016/j.brainres.2019.146302. [DOI] [PubMed] [Google Scholar]
- 52.Yang E, Wang X, Gong Z, Yu M, Wu H, Zhang D. Exosome-mediated metabolic reprogramming: the emerging role in tumor microenvironment remodeling and its influence on cancer progression. Signal Transduct Target Ther . 2020;5:242. doi: 10.1038/s41392-020-00359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bao S, Tang F, Li X, Hayashi K, Gillich A, Lao K, Surani MA. Epigenetic reversion of post-implantation epiblast to pluripotent embryonic stem cells. Nature . 2009;461:1292–1295. doi: 10.1038/nature08534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Downing TL, Soto J, Morez C, Houssin T, Fritz A, Yuan F, Chu J, Patel S, Schaffer DV, Li S. Biophysical regulation of epigenetic state and cell reprogramming. Nat Mater . 2013;12:1154–1162. doi: 10.1038/nmat3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Citron F, Fabris L. Targeting Epigenetic Dependencies in Solid Tumors: Evolutionary Landscape Beyond Germ Layers Origin. Cancers (Basel) . 2020;12:682. doi: 10.3390/cancers12030682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eckschlager T, Plch J, Stiborova M, Hrabeta J. Histone Deacetylase Inhibitors as Anticancer Drugs. Int J Mol Sci . 2017;18 doi: 10.3390/ijms18071414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perla A, Fratini L, Cardoso PS, Nör C, Brunetto AT, Brunetto AL, de Farias CB, Jaeger M, Roesler R. Histone Deacetylase Inhibitors in Pediatric Brain Cancers: Biological Activities and Therapeutic Potential. Front Cell Dev Biol . 2020;8:546. doi: 10.3389/fcell.2020.00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reddy RG, Bhat UA, Chakravarty S, Kumar A. Advances in histone deacetylase inhibitors in targeting glioblastoma stem cells. Cancer Chemother Pharmacol . 2020;86:165–179. doi: 10.1007/s00280-020-04109-w. [DOI] [PubMed] [Google Scholar]
- 59.Staberg M, Michaelsen SR, Rasmussen RD, Villingshøj M, Poulsen HS, Hamerlik P. Inhibition of histone deacetylases sensitizes glioblastoma cells to lomustine. Cell Oncol (Dordr) . 2017;40:21–32. doi: 10.1007/s13402-016-0301-9. [DOI] [PubMed] [Google Scholar]
- 60.Johnstone RW. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov . 2002;1:287–299. doi: 10.1038/nrd772. [DOI] [PubMed] [Google Scholar]
- 61.da Cunha Jaeger M, Ghisleni EC, Cardoso PS, Siniglaglia M, Falcon T, Brunetto AT, Brunetto AL, de Farias CB, Taylor MD, Nör C, Ramaswamy V, Roesler R. HDAC and MAPK/ERK Inhibitors Cooperate To Reduce Viability and Stemness in Medulloblastoma. J Mol Neurosci . 2020;70:981–992. doi: 10.1007/s12031-020-01505-y. [DOI] [PubMed] [Google Scholar]
- 62.Coni S, Mancuso AB, Di Magno L, Sdruscia G, Manni S, Serrao SM, Rotili D, Spiombi E, Bufalieri F, Petroni M, Kusio-Kobialka M, De Smaele E, Ferretti E, Capalbo C, Mai A, Niewiadomski P, Screpanti I, Di Marcotullio L, Canettieri G. Selective targeting of HDAC1/2 elicits anticancer effects through Gli1 acetylation in preclinical models of SHH Medulloblastoma. Sci Rep . 2017;7:44079. doi: 10.1038/srep44079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meel MH, de Gooijer MC, Metselaar DS, Sewing ACP, Zwaan K, Waranecki P, Breur M, Buil LCM, Lagerweij T, Wedekind LE, Twisk JWR, Koster J, Hashizume R, Raabe EH, Montero Carcaboso Á, Bugiani M, Phoenix TN, van Tellingen O, van Vuurden DG, Kaspers GJL, Hulleman E. Combined Therapy of AXL and HDAC Inhibition Reverses Mesenchymal Transition in Diffuse Intrinsic Pontine Glioma. Clin Cancer Res . 2020;26:3319–3332. doi: 10.1158/1078-0432.CCR-19-3538. [DOI] [PubMed] [Google Scholar]
- 64.Shofuda T, Kanemura Y. HDACs and MYC in medulloblastoma: how do HDAC inhibitors control MYC-amplified tumors? Neuro Oncol . 2021;23:173–174. doi: 10.1093/neuonc/noaa292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Orzan F, Pellegatta S, Poliani PL, Pisati F, Caldera V, Menghi F, Kapetis D, Marras C, Schiffer D, Finocchiaro G. Enhancer of Zeste 2 (EZH2) is up-regulated in malignant gliomas and in glioma stem-like cells. Neuropathol Appl Neurobiol . 2011;37:381–394. doi: 10.1111/j.1365-2990.2010.01132.x. [DOI] [PubMed] [Google Scholar]
- 66.Hsu CC, Chang WC, Hsu TI, Liu JJ, Yeh SH, Wang JY, Liou JP, Ko CY, Chang KY, Chuang JY. Suberoylanilide hydroxamic acid represses glioma stem-like cells. J Biomed Sci . 2016;23:81. doi: 10.1186/s12929-016-0296-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sung GJ, Kim SH, Kwak S, Park SH, Song JH, Jung JH, Kim H, Choi KC. Inhibition of TFEB oligomerization by co-treatment of melatonin with vorinostat promotes the therapeutic sensitivity in glioblastoma and glioma stem cells. J Pineal Res . 2019;66:e12556. doi: 10.1111/jpi.12556. [DOI] [PubMed] [Google Scholar]
- 68.Bubna AK. Vorinostat-An Overview. Indian J Dermatol . 2015;60:419. doi: 10.4103/0019-5154.160511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Subramanian S, Bates SE, Wright JJ, Espinoza-Delgado I, Piekarz RL. Clinical Toxicities of Histone Deacetylase Inhibitors. Pharmaceuticals (Basel) . 2010;3:2751–2767. doi: 10.3390/ph3092751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blumenschein GR Jr, Kies MS, Papadimitrakopoulou VA, Lu C, Kumar AJ, Ricker JL, Chiao JH, Chen C, Frankel SR. Phase II trial of the histone deacetylase inhibitor vorinostat (Zolinza, suberoylanilide hydroxamic acid, SAHA) in patients with recurrent and/or metastatic head and neck cancer. Invest New Drugs . 2008;26:81–87. doi: 10.1007/s10637-007-9075-2. [DOI] [PubMed] [Google Scholar]
- 71.Singh BN, Zhang G, Hwa YL, Li J, Dowdy SC, Jiang SW. Nonhistone protein acetylation as cancer therapy targets. Expert Rev Anticancer Ther . 2010;10:935–954. doi: 10.1586/era.10.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Patties I, Kortmann RD, Menzel F, Glasow A. Enhanced inhibition of clonogenic survival of human medulloblastoma cells by multimodal treatment with ionizing irradiation, epigenetic modifiers, and differentiation-inducing drugs. J Exp Clin Cancer Res . 2016;35:94. doi: 10.1186/s13046-016-0376-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lyko F, Brown R. DNA methyltransferase inhibitors and the development of epigenetic cancer therapies. J Natl Cancer Inst . 2005;97:1498–1506. doi: 10.1093/jnci/dji311. [DOI] [PubMed] [Google Scholar]
- 74.Zwergel C, Valente S, Mai A. DNA Methyltransferases Inhibitors from Natural Sources. Curr Top Med Chem . 2016;16:680–696. doi: 10.2174/1568026615666150825141505. [DOI] [PubMed] [Google Scholar]
- 75.Valente S, Liu Y, Schnekenburger M, Zwergel C, Cosconati S, Gros C, Tardugno M, Labella D, Florean C, Minden S, Hashimoto H, Chang Y, Zhang X, Kirsch G, Novellino E, Arimondo PB, Miele E, Ferretti E, Gulino A, Diederich M, Cheng X, Mai A. Selective non-nucleoside inhibitors of human DNA methyltransferases active in cancer including in cancer stem cells. J Med Chem . 2014;57:701–713. doi: 10.1021/jm4012627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zaba R. [Breath test using C-13-trioleate in the evaluation of the rate of fatty acid metabolism after parenteral feeding of premature and newborn infants] Pediatr Pol . 1988;63:661–664. [PubMed] [Google Scholar]
- 77.Andrade AF, Borges KS, Suazo VK, Geron L, Corrêa CA, Castro-Gamero AM, de Vasconcelos EJ, de Oliveira RS, Neder L, Yunes JA, Dos Santos Aguiar S, Scrideli CA, Tone LG. The DNA methyltransferase inhibitor zebularine exerts antitumor effects and reveals BATF2 as a poor prognostic marker for childhood medulloblastoma. Invest New Drugs . 2017;35:26–36. doi: 10.1007/s10637-016-0401-4. [DOI] [PubMed] [Google Scholar]