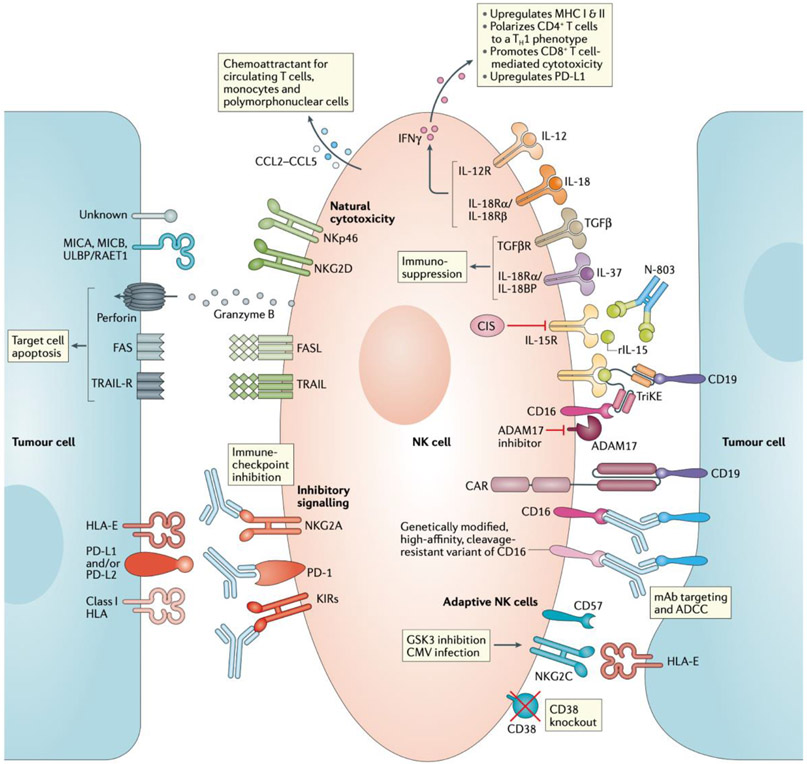
Fig. 2 ∣. Summary of the various approaches to enhancing NK cell effector function.
From top right, clockwise: pro-inflammatory cytokines such as IL-12 and IL-18 enhance NK cell effector function and cytokine secretion, whereas anti-inflammatory cytokines such as IL-37 and TGFβ suppress NK cell function. IL-15 is a crucial homeostatic cytokine for NK cells; cytokine-inducible SH2-containing protein (CIS) is a negative regulator of IL-15 signalling and thus a potential therapeutic target. Recombinant IL-15 (rIL-15) and the IL-15 superagonist N-803 promote NK cell proliferation and persistence within the tumour microenvironment. NK cell engagers (for example, tri-specific killer cell engagers (TriKEs) that also include an IL-15 domain to enhance NK cell activation) and monoclonal antibodies (mAbs) capable of binding to and activating CD16 (low-affinity IgG Fc region receptor III) on NK cells enable the targeting of these cells towards tumour cells expressing specific antigens, such as CD19. Disintegrin and metalloproteinase domain-containing protein 17 (ADAM17) inhibitors reduce CD16 cleavage from the cell surface and might, therefore, enhance NK cell activity. Genetically modified cell products, such as chimeric antigen receptor (CAR) NK cells or NK cells with modified forms of CD16 (for example, FT-516), are also being investigated as a means of improving the antitumour activity of adoptive NK cell therapies. Moreover, GSK3 inhibition and cytomegalovirus (CMV) infection give rise to hyperfunctional NKG2C+CD57+ adaptive NK cells, which have been associated with favourable clinical outcomes. Knockout of CD38 in adoptively transferred NK cells can prevent antibody-dependent cell-mediated cytotoxicity (ADCC)-related fratricide during treatment with mAbs targeting this protein, such as daratumumab. Immune- checkpoint inhibitors could potentially relieve suppression of NK cell- mediated cytotoxicity by preventing inhibitory signalling through PD-1, NKG2A and killer immunoglobulin-like receptors (KIRs). Ultimately, signalling induced by Fas ligand (FASL) and TNF-related apoptosis-inducing ligand (TRAIL) on NK cells can trigger apoptosis of target cells. In addition, activation of members of the natural cytotoxicity receptor family, such as NKp46 and NKG2D, results in the release of preformed cytolytic granules containing granzyme B and perforin. NK cells also secrete chemokines that attract multiple subsets of immune cells with potential antitumour functions.