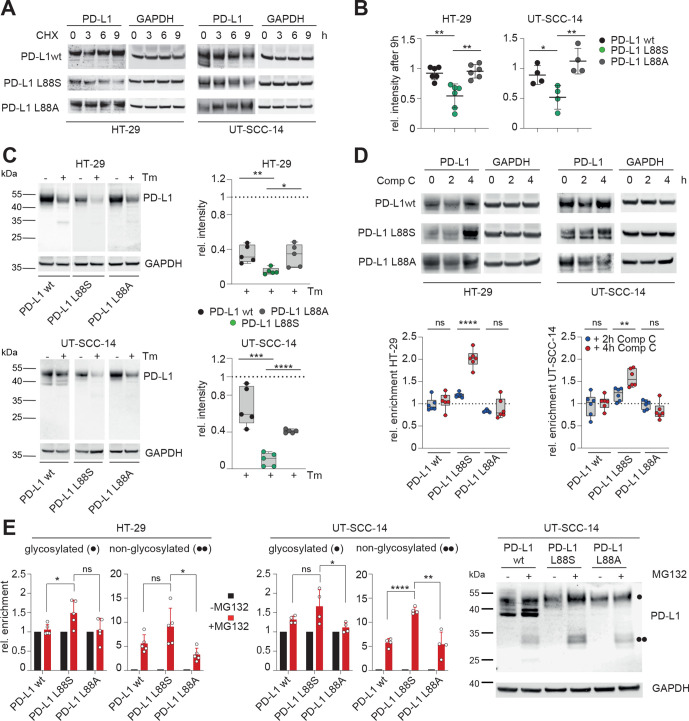
Figure 6.
PD-L1 L88S exhibits enhanced phosphorylation-dependent proteasomal degradation. (A) immunoblot analysis of PD-L1 abundancy in HT-29 (n=6) and UT-SCC-14 (n=4) cells overexpressing PD-L1 variants after blocking protein synthesis with 20 µM CHX for 3, 6 and 9 hours (h). (B) Quantification of (A) and four replicates using ImageJ. (C) Relative stability of PD-L1 protein (=signal intensity relative to control as quantified using ImageJ) in PD-L1 overexpressing cells after abrogation of N-glycosylation for 18 hours using tunicamycin (Tm) as determined by immunoblotting (n=5). (D) Quantification of PD-L1 protein abundancy in PD-L1 overexpressing cells after 2-hour and 4-hour blocking of AMPK with 10 µM compound C (Comp C) as determined by immunoblotting (n=6). (E) Enrichment of PD-L1 variants after inhibition of the proteasome using 20 µM MG132 for 4 hours. Quantification of glycosylated (·) and non-glycosylated (··) PD-L1 from four replicates of PD-L1 transduced HT-29 and UT-SCC-14 cells. Statistics: two-tailed unpaired t-test. Asterisks indicate p value range (*p<0.05; **p<0.01; ***p<0.001; ***p<0.0001; ns>0.05). AMPK, AMP-activated protein kinase; PD-L1, programmed cell death protein ligand 1.