Table 1.
Inhibition of Fluorescent Antagonist (2) Binding in hP2Y14R-Expressing CHO Cells (X, Y, and Z = CH, Unless Noted)
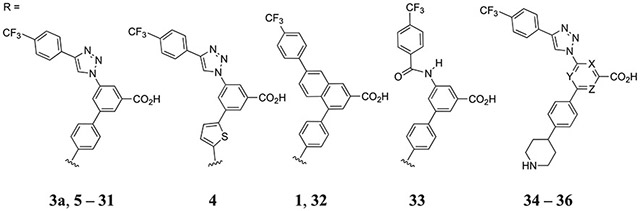 | |||
|---|---|---|---|
| Compound | Structurea = | IC50, hP2Y14R (nM, mean±SEM) |
cLogPc |
| Archival compounds and close analogues | |||
| 1ab | 7.96±3.5 | 6.18 | |
| 1bb | 27.6±4.3 | 6.47 | |
| 1c | 36.6±10.9 | 5.99 | |
| 1d | 240±21 | 6.70 | |
| 3ab | 31.7±8.0 | 4.64 | |
| 3b | 60,500±6900 | 3.89 | |
| 4b | R-CONH(CH2)3NH2 | 169±42 | 0.84 |
| 5b | R-CONH2 | 269±121 | 3.63 |
| 6b | 233±26 | 3.95 | |
| Phenyl-CO or NH | |||
| 7 | R-NH2 | 1480±160 | 4.03 |
| 8 | R-NH-Ac | 811±95 | 4.07 |
| 9 | R-NH-CO-phenyl | 1860±540 | 5.19 |
| 10 | R-NH-Boc | 3160±310 | 4.98 |
| 11 | 197±29 | 3.85 | |
| 12 | 22,500±3700 | 4.85 | |
| 13 | R-COOH | 632±23 | 3.88 |
| 14 | R-CONH(CH2)3NH2 | 588±43 | 0.92 |
| Phenyl-halo or acyclic | |||
| 15 | R-Br | >10,000 | 4.71 |
| 16 | R-CH2CONH2 | 1290±320 | 3.65 |
| 17 | R-(CH2)2-CN | 17,800±2900 | 4.74 |
| 18 | R-(CH2)3-NH2 | 292±67 | 1.60 |
| 19 | R-C≡CCH2-NH2 | 308±63 | 1.47 |
| 20 | R-(CH2)3-NH-Boc | 42,500±27,000 | 5.37 |
| 21 | R-(CH2)4-OH | 9220±1220 | 4.84 |
| Phenyl-cyclic | |||
| 22 | 14,100±900 | 1.36 | |
| 23 | 8910±750 | 1.57 | |
| 24 | 18,800±2000 | 5.41 | |
| 25 | 2000±180 | 1.70 | |
| 26 | >10,000 | 5.41 | |
| 27 | 2860±440 | 4.73 | |
| 28 | 2230±380 | 4.98 | |
| 29 | 296±13 | 4.44 | |
| 30 | 389±42 | 4.59 | |
| 31 | 895±122 | 3.65 | |
| Alternative scaffolds | |||
| 32 | 15.0±144 | 5.77 | |
| 33 | 7400±1050 | 4.36 | |
| Aza-scan of 3a | |||
| 34 | 1690±280 | 4.16 | |
| 35 | 4900±540 | 4.10 | |
| 36 | 2920±310 | 4.01 | |
