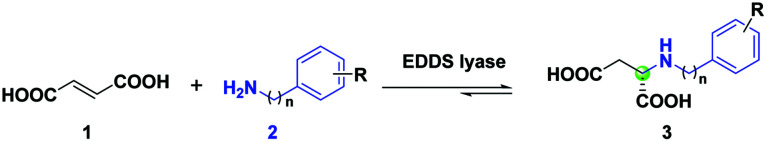
Semi-preparative-scale synthesis of N-arylalkyl-substituted l-aspartic acids via EDDS lyase catalyzed enantioselective hydroaminationa.
| ||||||
|---|---|---|---|---|---|---|
| Entry | Amine | Product | Reaction time (h) | Yieldb (%) (mg) | eec (%) | Abs. conf.d |
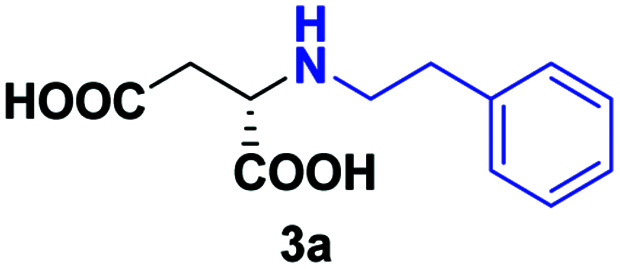
| 1 | 2a |
|
24 | 70 (25) | >99 | S |
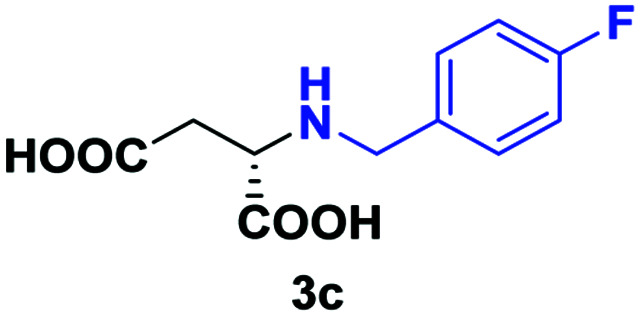
| 2 | 2c |
|
48 | 75 (27) | >99 | S |
| 3 | 2d |

|
24 | 28 (11) | >99 | S |
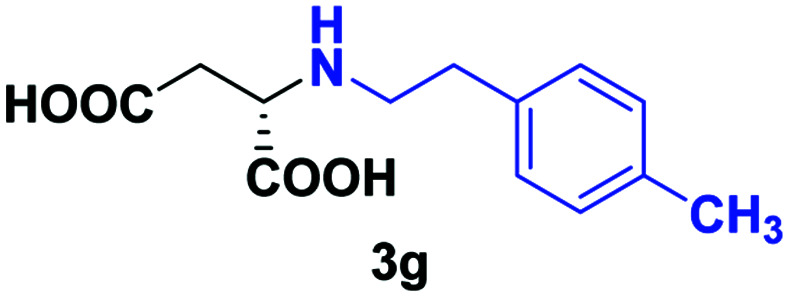
| 4 | 2g |
|
24 | 66 (25) | >99 | S |
| 5 | 2h |

|
24 | 61 (23) | >99 | S |
| 6 | 2i |

|
24 | 63 (24) | >99 | S |
| 7 | 2j |

|
24 | 76 (29) | >99 | S |
Reaction mixtures contained 1 (10 mM, 0.15 mmol), 2a, 2c, 2d or 2g–j (50 mM), and EDDS lyase (15 μM) in 15 ml NaH2PO4 buffer (20 mM, pH 8.5, room temperature).
Isolated product yield after ion-exchange chromatography.
The ee was determined by high-performance liquid chromatography (HPLC) using a chiral stationary phase and chemically synthesized authentic standards.
Determined by HPLC using a chiral stationary phase and chemically synthesized authentic standards.
