Figure 1.
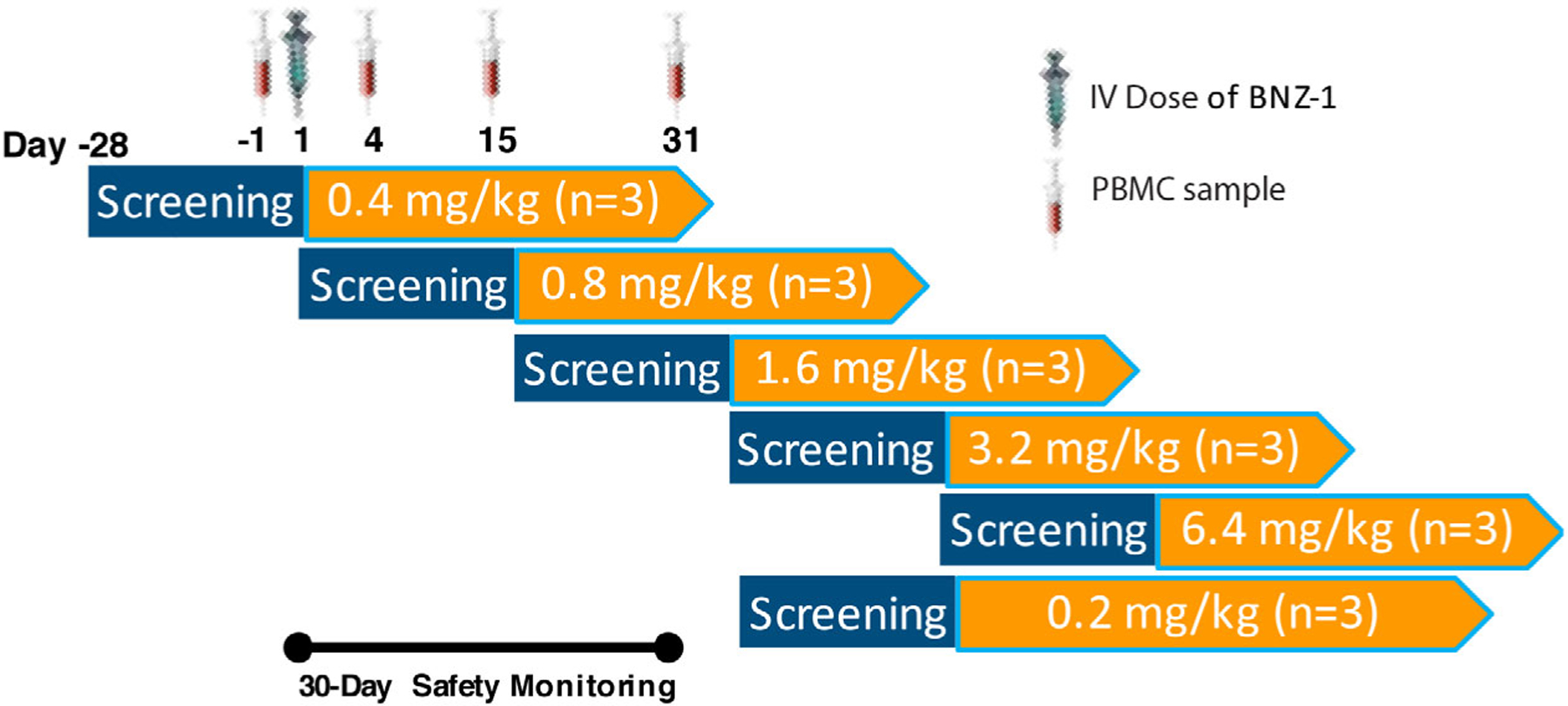
The first-in-human study included a 4-week screening period, a single IV dose of BNZ-1 administered on day 1, and a 30-day safety monitoring and sample collection period. PBMC samples for flow cytometry were collected before dosing and 3, 14, and 30 days after dosing. The starting dose of 0.4 mg/kg was estimated to be a no-effect dose based on preclinical data. However, 0.4 mg/kg was shown to have significant pharmacodynamic activity, so a lower dose of 0.2 mg/kg was added during the study to try and identify a no-effect dose. IV, intravenous; PBMC, peripheral blood mononuclear cell.
