Scheme 1.
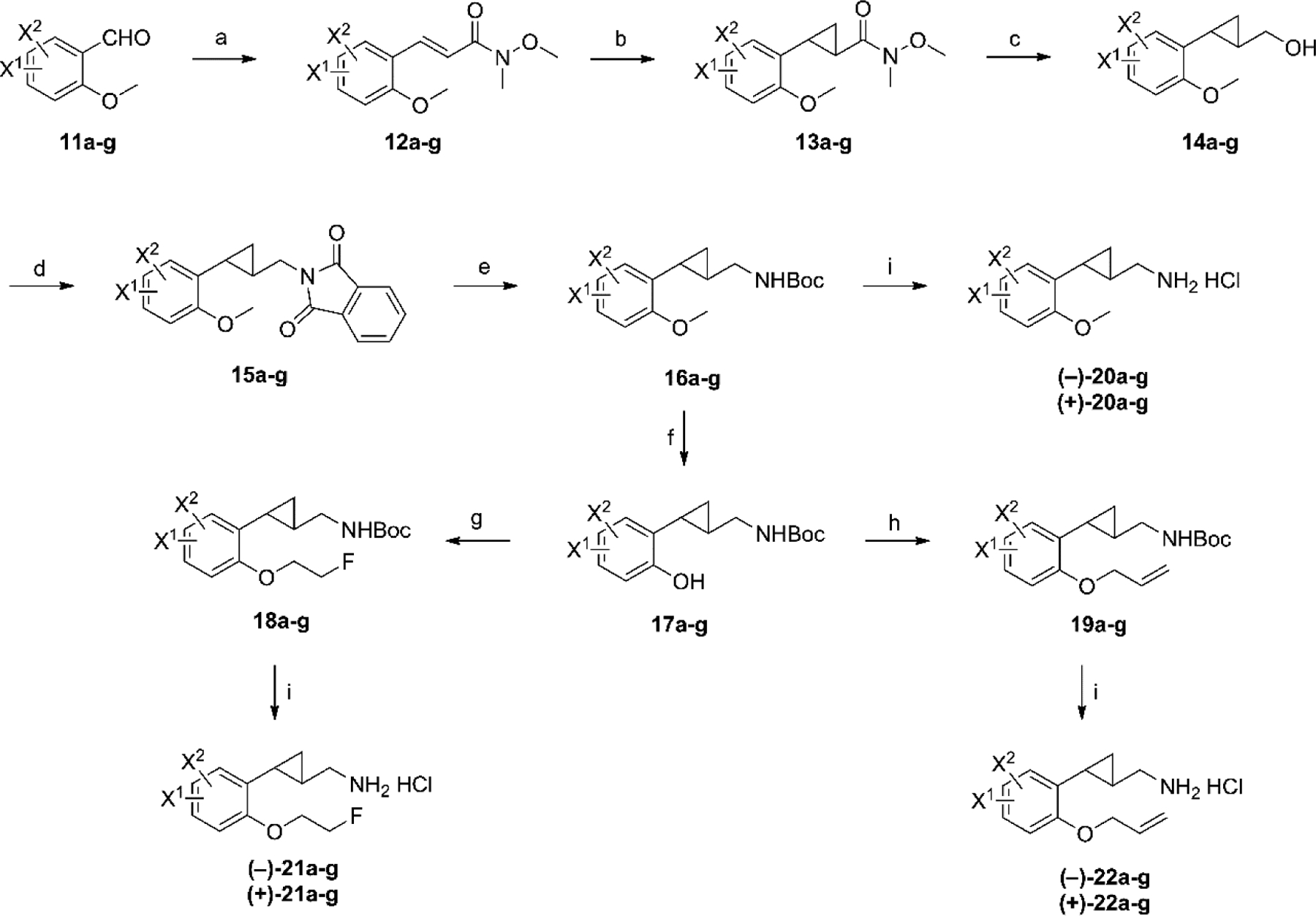
Synthesis of target compounds 20a–g, 21a–g, and 22a–g.a a Reagents and conditions: (a) Ph3P=CHC(O)N(OMe)Me, CH2Cl2, rt, overnight; (b) Me3S+(O)I−, NaH, DMSO, rt, overnight; (c) DIBAL-H, THF, −78 °C, 2 h; then NaBH4, MeOH, 0 °C to rt, 0.5 h; (d) phthalimide, PPh3, DEAD, THF, rt, overnight; (e) N2H4−H2O, EtOH, reflux, 3h; then Boc2O, Et3N, CH2Cl2, rt, 0.5 h; (f) BBr3, CH2Cl2, −78 °C to rt; then Boc2O, Et3N, CH2Cl2; (g) 2-fluoroethanol, Ph3P, DEAD, THF, 0 °C to 60 °C, 1h; (h) allyl bromide, Cs2CO3, DMF; (i) (1) chiral preparative-HPLC; (2) 2M HCl in Et2O, rt, 24 h. 11–22a: 4, 5-diF; 11–22b: 5, 6-diF; 11–22c: 4-F, 5-Cl; 11–22d: 5-Cl, 6-F; 11–22e: 4, 5-diCl; 11–22f: 5, 6-diCl; 11–22g: 5-F, 6-Cl.
