Scheme 7. Inverted Chemoselectivity for Cross-Cycloadditions of Vinylsilanes with 2-Substituted-1,3-Dienes.a.
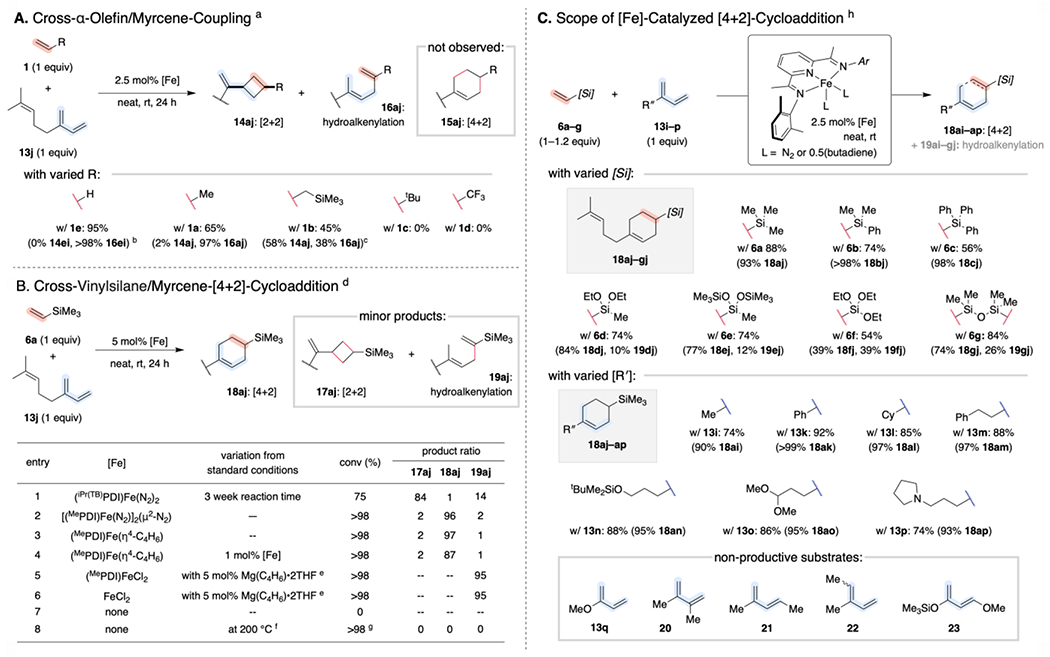
a Reactions conducted on 0.25 or 0.5 mmol scale. Combined isolated yields reported (with selectivity for each product listed in parentheses). b Reaction conducted in C6D6 using isoprene (13i) in place of myrcene (13j) in C6D6. Yield (and selectivity for hydrovinylation product 16ei) determined by integration of diagnostic 1H NMR signals relative to an internal standard. c With 5 mol% [Fe] d Reactions conducted on 0.25 mmol scale. Conversion and product ratio determined after 24 h by gas chromatographic analysis relative to mesitylene, which was added as an internal standard at the end of the reaction. e 5 mol% Mg(butadiene)•2THF added as an in situ reductant. f Reaction performed on a 1.9 mmol scale at 200 °C for 24 h with 5 mg BHT. g Complex mixture of decomposition products. h Reactions conducted on 1.0 mmol scale. Combined isolated yields reported (with selectivity for each product listed in parentheses).
