Scheme 5. Conserved Chemoselectivity for Cross-Cycloadditions with 4-Substituted-1,3-Dienes.
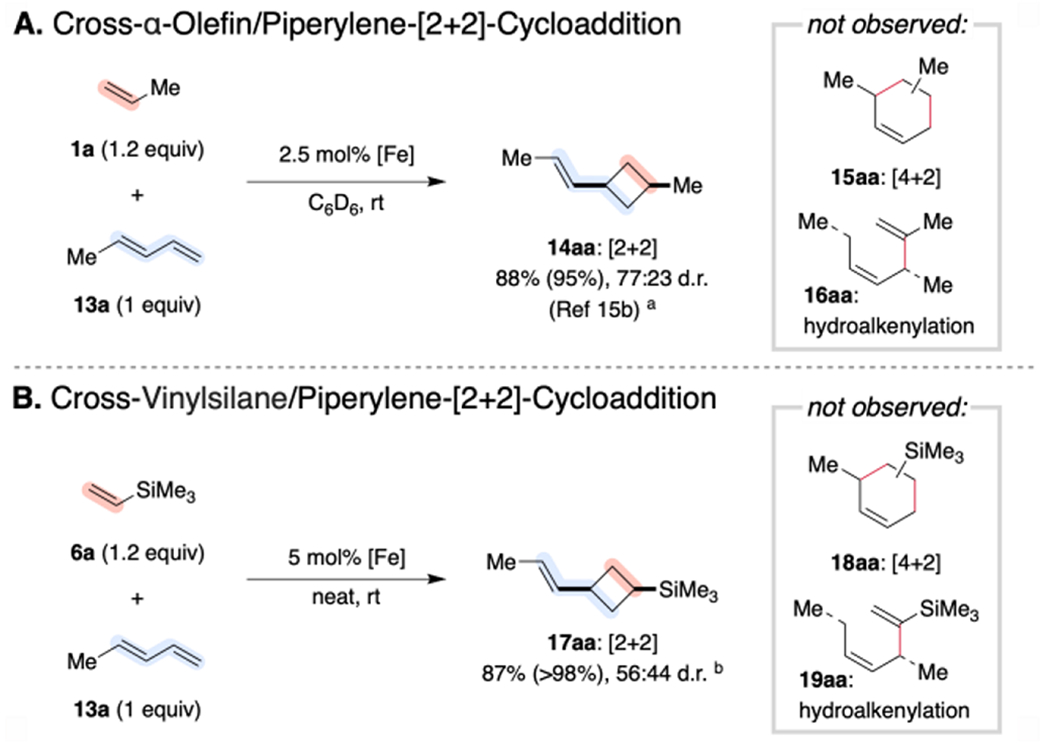
a Conducted on 1.0 mmol scale with 1.25 mol% [(Me(Et)PDI)Fe(N2)]2(μ2-N2). Yield (and selectivity for [2+2]-cycloadduct 14aa) determined by integration of diagnostic 1H NMR signals relative to an internal standard. See Ref. 15b. b Conducted on 1.0 mmol scale with 2.5 mol% [(MePDI)Fe(N2)]2(μ2-N2). Isolated yield reported (with selectivity for the [2+2]-cycloadduct 17aa product in parentheses).
