Abstract
Context
Inhibitors of the protease neprilysin (NEP) are used for treating heart failure, but are also linked to improvements in metabolism. NEP may cleave proglucagon-derived peptides, including the glucose and amino acid (AA)-regulating hormone glucagon. Studies investigating NEP inhibition on glucagon metabolism are warranted.
Objective
This work aims to investigate whether NEP inhibition increases glucagon levels.
Methods
Plasma concentrations of glucagon and AAs were measured in eight healthy men during a mixed meal with and without a single dose of the NEP inhibitor/angiotensin II type 1 receptor antagonist, sacubitril/valsartan (194 mg/206 mg). Long-term effects of sacubitril/valsartan (8 weeks) were investigated in individuals with obesity (n = 7). Mass spectrometry was used to investigate NEP-induced glucagon degradation, and the derived glucagon fragments were tested pharmacologically in cells transfected with the glucagon receptor (GCGR). Genetic deletion or pharmacological inhibition of NEP with or without concomitant GCGR antagonism was tested in mice to evaluate effects on AA metabolism.
Results
In healthy men, a single dose of sacubitril/valsartan significantly increased postprandial concentrations of glucagon by 228%, concomitantly lowering concentrations of AAs including glucagonotropic AAs. Eight-week sacubitril/valsartan treatment increased fasting glucagon concentrations in individuals with obesity. NEP cleaved glucagon into 5 inactive fragments (in vitro). Pharmacological NEP inhibition protected both exogenous and endogenous glucagon in mice after an AA challenge, while NEP-deficient mice showed elevated fasting and AA-stimulated plasma concentrations of glucagon and urea compared to controls.
Conclusion
NEP cleaves glucagon, and inhibitors of NEP result in hyperglucagonemia and may increase postprandial AA catabolism without affecting glycemia.
Keywords: Entresto, (sacubitril/valsartan), proglucagon-derived peptides, metabolism
The endopeptidase neprilysin (NEP) is widely expressed in humans [1, 2], and several peptide hormones including the vasoactive hormone, atrial natriuretic peptide (ANP), and the proglucagon-derived incretin hormone, glucagon-like peptide 1 (GLP-1), have been identified as substrates [3-6]. Inhibitors of NEP (sacubitril) combined with the angiotensin II type 1 receptor antagonist (valsartan) are currently used in the treatment of chronic heart failure [7], but have recently also been reported to improve metabolic function [6, 8]. The proglucagon-derived hormone glucagon is a substrate for NEP degradation in vitro [9, 10] and NEP degrades both exogenous and endogenous glucagon in pigs [11]. Glucagon plays a central role in hepatic amino acid (AA) metabolism, both at the transcriptional level and during increased AA availability [12, 13]. AAs stimulate the secretion of glucagon, and glucagon in turn regulates hepatic AA catabolism by stimulating ureagenesis [12, 14-23]. The metabolic implications of NEP inhibitors on endogenous glucagon levels and AA metabolism are currently unknown.
We hypothesized that NEP cleaves glucagon and that inhibitors of NEP increase AA catabolism in a glucagon receptor (GCGR)-dependent manner. To address this, we measured plasma concentrations of glucagon and AAs in healthy young men and individuals with obesity after administration of the dual NEP inhibitor/angiotensin II type 1 receptor antagonist, sacubitril/valsartan, because sacubitril alone is not available for use in humans. Moreover, we examined the effects of pharmacological NEP inhibition as well as genetic deficiency of NEP activity in mice. To dissect the metabolic effects of NEP inhibition, we modified GCGR signaling, using a GCGR antagonist (GRA). To account for the potential effects of altered GLP-1 receptor (GLP-1R) signaling on inhibition of NEP, we also studied mice treated with a GLP-1R antagonist (Ex9-39) or mice lacking the GLP-1R (GLP-1R–/–). Finally, we assessed NEP-induced glucagon degradation in vitro, and examined potential agonistic properties of the generated glucagon fragments for GCGR activity in COS-7 cells transiently transfected with human or murine GCGR.
Materials and Methods
Study Approvals and Ethical Considerations
The study of healthy young men was approved by the scientific-ethical committee of the Capital region of Denmark (H-18000360) and registered with the Danish Data Protection Agency (VD-2018-131) and ClinicalTrials.gov (NCT03717688). The long-term study investigating individuals with obesity was conducted by the Maastricht University Medical Centre and was approved by the institutional review board or the independent ethics committee for each participating center, and registered at clinicaltrials.gov (NCT01631864). Written and oral consent was obtained from all participants, and both studies were conducted in accordance with the principles of the Declaration of Helsinki. Primary outcomes from both cohorts have previously been reported [5, 24]. Animal experiments were conducted with permission from the Danish Animal Experiments Inspectorate, Ministry of Environment and Food of Denmark (2018-15-0201-01397), VA Puget Sound Health Care system Institutional Animal Care and Use Committee (IACUC) in Seattle, Washington, USA, and the Department of Experimental Medicine IACUC in Copenhagen, Denmark (approval Nos. P19-147 and P19-475). All studies were performed in accordance with the European Union Directive 2010/63/EU.
Clinical Study Designs and Participants
The acute effect of sacubitril/valsartan was investigated in healthy men as a crossover trial. Eight men received in randomized order a single dose (2 tablets) of the NEP-inhibitor/angiotensin II type 1 receptor antagonist (194 mg sacubitril/206 mg valsartan) (Novartis Pharma GmbH) 30 minutes before ingesting a solid mixed meal (42 g/34% carbohydrates, 25 g/45% fat, 26 g/21% protein) or the meal alone. Participants were studied in the morning after an overnight (10-hour) fast, and the meal was ingested over a 10-minute period. A cannula was inserted into a cubital vein and blood samples were drawn at –60 minutes (fasting) and –30, –15, 0, 15, 30, 45, 60, 90, 120, 180, and 240 minutes relative to meal ingestion (at time 0 minutes). After completion of the study, 3 participants meeting the same inclusion criteria were administered valsartan (206 mg) alone 30 minutes before ingesting the same solid mixed meal previously described or the meal alone (at time 0 minutes), with blood drawn at times –60, –15, 0, 30, 45, 90, and 180 minutes. Long-term effects of sacubitril/valsartan were investigated in individuals with hypertension and obesity in a multicenter, randomized, double-blinded study. Participants were administered 8 weeks of treatment of either sacubitril/valsartan (194 mg/206 mg once a day, n = 7) or amlodipine, included in this study as a control group (10 mg once a day, n = 7). Plasma samples were obtained after an overnight fast before and after 8 weeks treatment.
Animals and Compounds
Female and male C57BL/6JRj mice (20-25 g) were acquired from Janvier Laboratories. Female mice deficient in GLP-1R signaling (GLP-1R–/–; C57BL/6N-Glp1rtm1c(KOMP)MbpH) and wild-type littermates (GLP-1R+/+) (previously characterized [25]) were bred in house. Experiments on male mice deficient in NEP activity (NEP–/–) (C57BL/6.NEP–/–), acquired from Dr B. Lu (Harvard Medical School [26]) with subsequent establishment in Seattle, Washington, USA [27], were conducted at the University of Washington, Seattle. All mice were housed in groups of 3 to 8 in individually ventilated cages, following a light-dark cycle of 12 hours (lights on from 6 am to 6 pm) with ad libitum access to standard chow (Altromin Spezialfutter) and water. Mice were acclimatized for a minimum of 1 week after transferal before being included in the experiments. A GCGR antagonist (GRA) (100 mg/kg body weight [BW]) (25-2648; a gift from Novo Nordisk [28]) was administered in suspension by oral gavage as previously described [29, 30]. The NEP inhibitor, sacubitril (dissolved in dimethyl sulfoxide [DMSO] and diluted in phosphate-buffered saline [PBS]) was administered by oral gavage at a concentration (0.7 nmol/g BW) corresponding to 1000 times the half-maximal inhibitory concentration (IC50) for sacubitril [31] after correcting for 60% bioavailability. PBS (0.7% DMSO) was administered by oral gavage as a sacubitril control. The GLP-1R antagonist, Ex9-39 (Bachem), was administered as a fixed dose of 100 µg by intraperitoneal injection. Glucagon (96 ng/g BW; Bachem), dissolved in DMSO and diluted in PBS + 0.1% bovine serum albumin, was administered by intraperitoneal injection. AAs were administered by oral gavage (Vamin 14 g/L Electrolyte Free; Fresenius Kabi).
Study Design for Mouse Experiments
First, we investigated the effects of sacubitril on exogenous glucagon concentrations in female C57BL/6JRj mice that received either sacubitril or vehicle 30 minutes prior to an injection of glucagon at time 0 minutes. Blood glucose concentrations were measured from tail bleeds using a handheld glucometer (Accu-Chek Mobile; Roche Diagnostics), and blood was collected in EDTA-coated capillary tubes (micro haematocrit tubes; Vitrex Medical) at time points 0, 5, 15, 30, and 60 minutes. Next, we investigated the effects of the NEP inhibitor on endogenous glucagon concentrations in female (n = 7-10) and male (n = 6) C57BL/6JRj mice (~ 22 g) treated with GRA vehicle (time –180 minutes), GRA (time –180 minutes), or Ex9-39 (time –15 minutes). Sacubitril or vehicle were administered at time –30 minutes followed by a fixed dose of AAs (34 mg) at time 0 minutes. Blood glucose concentrations and blood samples were collected and stored as described earlier at times 0, 10, 15, 30, 60, and 180 min. The experiment was also repeated in female mice that received water instead of AAs (n = 4). GLP-1R–/– mice and GLP-1R+/+ mice were treated with sacubitril or vehicle at time –30 minutes followed by an AA challenge (34 mg), with measurement of blood glucose and collection of blood at times 0, 10, 15, 30, 60, and 180 minutes, as described earlier. Male NEP–/– mice and nonlittermate controls (NEP+/+) (n = 5-6) were administered a volume of AAs equivalent to 1% of BW (corresponding to ~ 22 mg). Blood glucose concentrations were measured with a glucometer (Accu-Chek Aviva Plus; Roche) and blood was drawn in heparinized hematocrit tubes (Fisherbrand micro-hematocrit capillary tubes) at times 0, 15, 30, and 60 minutes. All blood samples from the aforementioned studies were collected from the retro bulbar plexus (~ 75 µL) and kept on ice until centrifugation with plasma being transferred to prechilled PCR tubes and stored at –20 °C or –80 °C for subsequent analyses. All mice were fasted for 3 hours prior to experiments with water freely accessible. Mice were euthanized by cervical dislocation.
In Vitro Degradation of Glucagon by Neprilysin and Pharmacological Characterization of Glucagon-Degradation Products
NEP-induced glucagon degradation was investigated in vitro using synthetic glucagon (Bachem) and recombinant human or mouse (catalog Nos. 1182-ZNC-010 and 1126-ZNC-010; R&D Systems) NEP. A total of 10 ng recombinant NEP was added to 1 µg synthetic glucagon with or without the NEP inhibitor in the active form of the prodrug, sacubitril (sacubitrilat, 0.5 nmol, 10% DMSO) (catalog No. SML2064, Merck). Reactions were performed in 50 mM Tris, 0.05% Brij-35, pH 9 (human-derived NEP), or 50 mM Tris, pH 9 (mouse-derived NEP) and incubated at 37 °C. The reaction was stopped after 15, 30, or 60 minutes’ incubation by addition of an excess of 0.1% trifluoroacetic acid and spotted onto a ground steel matrix-assisted laser desorption/ionization (MALDI) target plate using α-cyano-4-hydroxycinnamic acid as a matrix. The mass spectrometer was calibrated for each reaction. Metabolite generation was assessed by a Bruker AutoFlex MALDI–time of flight (TOF) mass spectrometer with accompanying Compass version 1.4 FlexSeries software. Synthetic ANP (catalog No. 005-06; Phoenix Pharmaceuticals) was included as a positive control because this peptide is a known substrate for NEP [32, 33]. A computation tool (web.expasy.org/compute) was used to find AA sequences corresponding to the molecular weight of the identified degradation products. AA sequences were synthesized (CASLO ApS) and tested on COS-7 cells transfected with human or mouse GCGR to assess potential activation of GCGR signaling, antagonism (evaluated as 3′,5′-cyclic adenosine 5′-monophosphate [cAMP] production) and binding affinity (measured by displacement of [125I]-glucagon). COS-7 cells were cultured as previously described [34] and transiently transfected using the calcium phosphate precipitation method [35]. To test for agonism, the glucagon-degradation products were added in increasing concentrations (10 pM-10 µM) to the transfected COS-7 cells. Human glucagon (hGCG-NH2) was used as a positive control. To test for antagonistic properties, COS-7 cells were preincubated with increasing concentrations of the degradation products (0.1 nM, 10 nM, and 1 µM), with subsequent addition of the agonist (1 nM glucagon corresponding to 40% of the maximal cAMP accumulation response to glucagon). cAMP production was measured with HitHunter cAMP XS assay (DiscoverX) according to the manufacturer’s instructions. Finally, to test for competition binding, COS-7 cells were seeded in 96-well plates and incubated with 15 to 40 pM monoiodinated [125I]-labeled glucagon and different concentrations of the glucagon degradation products (1 pM-10 µM). Specific binding was calculated by subtracting the nonspecific binding from total binding. Samples were analyzed using the Wallac Wizard 1470 Gamma Counter and the output was calculated as the percentage of specific binding. Unlabeled synthetic glucagon (0.1 pM-1 µM) (catalog No. H-6790; Bachem) was used as a positive control.
Biochemical Analyses
Plasma concentrations of glucagon were measured by a validated [36] 2-site enzyme immunoassay (Mercodia catalog No. 10-1281-01, RRID:AB_2783839), recognizing both COOH- and NH2-termini of the molecule, according to the manufacturer’s protocol, and by an in-house radioimmunoassay (RIA) specific for the COOH-terminus of glucagon, using antiserum (no. 4305) and monoiodinated [125I]-labeled glucagon, as previously described [37, 38]. Plasma concentrations of insulin (Mercodia catalog No. 10-1247-01, RRID:AB_2783837), L-AAs (Abcam), and urea (BioAssay Systems) were measured according to manufacturers’ protocol. Samples for measuring individual AAs were derivatized with methyl chloroformate and measured using a slightly modified version of the previously described protocol [39], and processed as previously described [40].
Statistics
Incremental and total areas under the curve (iAUC and tAUC) were calculated using the trapezoidal rule, with iAUC calculated as positive peaks after adjusting for baseline values and tAUC calculated as positive peaks above 0. IC50 and EC50 values were determined by nonlinear regression. Significance was assessed by unpaired t tests, paired t tests, one-way analysis of variance or 2-way analysis of variance/mixed-effects analysis followed by Holm-Sidak post hoc analysis to correct for multiple testing. P less than or equal to .05 is considered significant and P equal to .05 to 0.1 is considered a trend. One symbol indicates P less than or equal to .05, 2 symbols indicate P than or equal to .01, and 3 symbols indicate P than or equal to .001. Statistical calculations and graphs were created using GraphPad Prism (version 8.01 for Windows; GraphPad Software). Data in the main text are reported as mean ± SD if not otherwise indicated.
Additional information on the methods for pharmacological characterization of glucagon-degradation products and biochemical analyses can be found in a digital research repository [41].
Results
Acute Neprilysin Inhibition Increases Plasma Concentrations of Glucagon and Reduces Amino Acids in Healthy Young Men
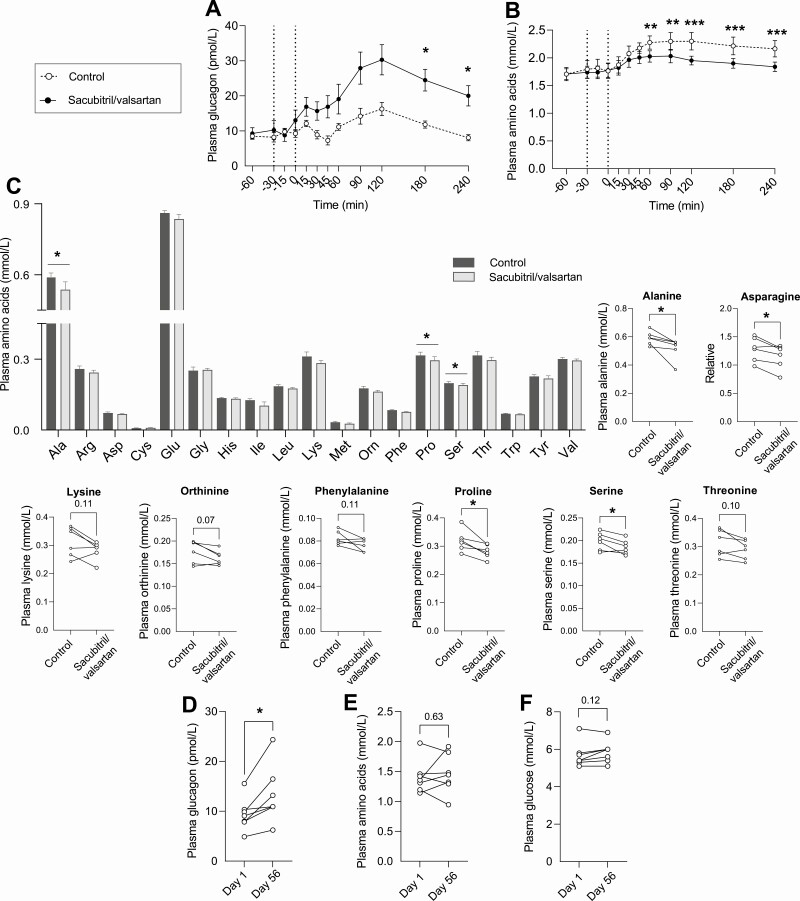
To investigate the effect of NEP inhibition on postprandial glucagon metabolism, healthy young men (mean ± SD; age, 24 ± 2 years, body mass index, 23 ± 1 kg/m2, n = 8) were included in a crossover study involving ingestion of a standardized solid high-fat mixed meal with or without coingestion of a single dose of the NEP inhibitor/angiotensin II type 1 receptor antagonist (sacubitril/valsartan). Sacubitril/valsartan did not significantly alter fasting concentrations of glucagon compared to control treatment (13 ± 8 vs 9 ± 3 pmol/L, P = .21), but postprandial glucagon concentrations were significantly increased by 228% compared to controls as reflected by iAUC (2435 ± 1455 vs 742 ± 365 pmol/L × min, P = .02). This was seen with both the sandwich enzyme-linked immunosorbent assay (ELISA) (employing NH2- and COOH-terminal wrapping antibodies; Fig. 1A) and the RIA (using a COOH-terminal wrapping antibody; Supplementary Fig. 1A [41]; all supplemental material and figures are available in a digital research repository [41]). Fasting and postprandial plasma concentrations of glucose were similar between control and sacubitril/valsartan treatments (Supplementary Fig. 1B [41]) (plasma glucose concentrations are previously published in [5]). Fasting AA concentrations were unchanged with sacubitril/valsartan (Fig. 1B), but postprandial AA concentrations were reduced by 12% compared to control treatment as reflected by tAUC (464 ± 55 vs 526 ± 81 mmol/L × min, P = .01), and plasma concentrations of glucagon (measured by ELISA) and AA were negatively correlated when evaluated by AUC (Supplementary Fig. 1C [41], P = .03). We also measured individual AAs at time point 90 minutes and found reduced concentrations of several AAs after treatment with sacubitril/valsartan (Fig. 1C, n = 6). Of particular note, levels of AAs (alanine, asparagine, and proline) known to be involved in the liver–α-cell axis were reduced concomitantly with the increase in plasma concentrations of glucagon. Because sacubitril is available for clinical use only in combination with valsartan, valsartan alone (206 mg) was administered to a subset of individuals (n = 3; Supplementary Fig. 1D [41]) to ascertain that the effects on glucagon were due to the inhibition of NEP activity and not to the changes in the renin-angiotensin-aldosterone system. Valsartan alone had no effect on postprandial plasma concentrations of glucagon when compared to control treatment as reflected by iAUC (1680 ± 428 vs 1744 ± 524 pmol/L × min, P = .55).
Figure 1.
Neprilysin (NEP) inhibition with sacubitril/valsartan increases plasma concentrations of glucagon in lean and obese individuals and reduces postprandial amino acid concentrations. Plasma concentrations of A, glucagon, and B, amino acids in healthy young males treated with sacubitril/valsartan (black circles) followed by a mixed meal (34% carbohydrates, 45% fat, and 21% protein) or the meal alone (controls, open circles) (cohort previously published in [5]). Sacubitril/valsartan was administered as a single dose (194 mg sacubitril/206 mg valsartan) 30 minutes before the meal (given at time 0 minutes). The same individuals completed the control and sacubitril/valsartan treatments in a randomized open-labeled crossover design (n = 8). C, Individual concentrations of amino acids in healthy men before control or sacubitril/valsartan treatment at time point 90 minutes after the meal (n = 6). Asparagine was not included in the column graph because of dissimilar y axes. Plasma concentrations of D, glucagon, E, amino acids, and F, glucose in individuals with obesity treated with sacubitril/valsartan for 8 weeks (n = 7) (cohort previously published in [24]). Data are presented as (B and C) mean ± SEM and are analyzed by (A and B) repeated-measures 2-way analysis of variance or C, D, E and F, paired t tests.
Long-Term Neprilysin Inhibition Increases Fasting Concentrations of Glucagon in Individuals With Obesity and Hypertension
The effect of long-term sacubitril/valsartan treatment on plasma concentrations of glucagon was investigated by analyzing plasma obtained before and after 8 weeks of treatment with sacubitril/valsartan in individuals with obesity and hypertension (1 woman; age, ~ 57 ± 7 years; body mass index, 33 ± 7 kg/m2;143/90 mm Hg, n = 7). Fasting concentrations of glucagon increased by 42% (9 ± 3 vs 13 ± 6 pmol/L, P = .02) (Fig. 1D), with no change in fasting concentrations of AAs (P = .63) (Fig. 1E) or glucose (P = .12) (Fig. 1F), consistent with the findings in the healthy young men in the fasted state. Seven individuals treated with amlodipine (calcium channel antagonist) for 8 weeks were included as controls and showed no significant changes in plasma concentrations of glucose, glucagon, or AAs (Supplementary Fig. 1E-1G [41]).
Recombinant Neprilysin Cleaves Native Glucagon In Vitro
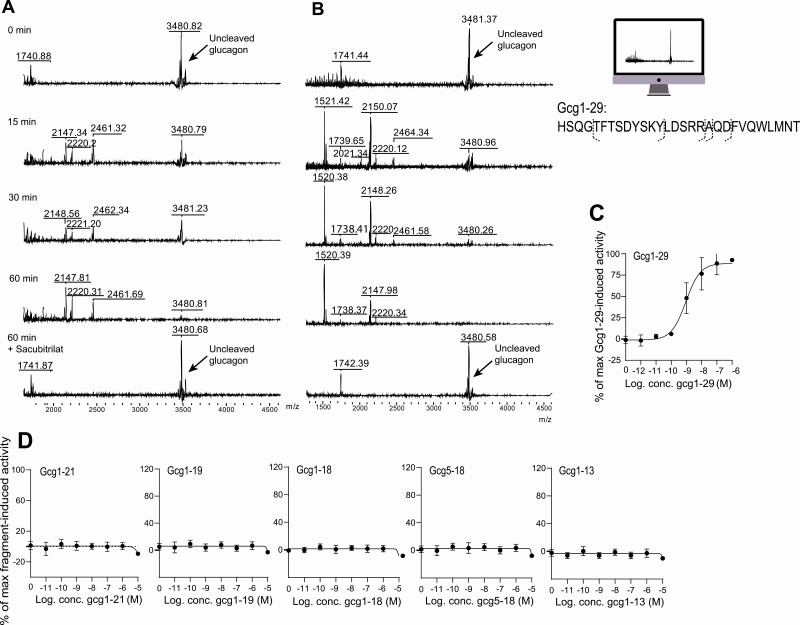
To investigate whether NEP degrades glucagon directly, native glucagon (Gcg1-29) was incubated with recombinant NEP with or without sacubitrilat (the active compound of the NEP inhibitor prodrug sacubitril), and products of degradation were assessed by MALDI-TOF mass spectrometry. As a control, we also analyzed glucagon in the absence of NEP and sacubitrilat, and observed that glucagon is stable over time (15, 30, and 60 minutes) (Fig. 2A and 2B “0 min”) when incubated in buffer alone. Recombinant human (see Fig. 2A) and mouse NEP (see Fig. 2B) cleaved native glucagon after 15 minutes’ incubation, as shown by the generation of 5 glucagon fragments and the reduced peak of native glucagon. The inclusion of sacubitrilat prevented the formation of any fragments and preserved the peak size of intact glucagon. Following analysis of the MALDI-TOF spectra, we identified NEP cleavage sites between AAs Asp21 and Phe22 (Gcg1-21), Ala19, and Gln20 (Gcg1-19), and Arg18 and Ala19 (Gcg1-18) in the COOH terminus of the glucagon molecule (Table 1). Two additional glucagon fragments were identified with recombinant mouse NEP with m/z values of 1738 and 1520, corresponding to AA sequences Gcg5-18 and Gcg1-13. These 2 glucagon fragments were not detectable following incubation of native glucagon with human recombinant NEP, probably because of a high signal-to-noise ratio at m/z less than 2000. ANP, a well characterized substrate of NEP [33, 42], was included as a positive control (Supplementary Fig. 2A and 2B [41]), and showed that NEP cleavage resulted in the formation of a known substrate (m/z 2585), which was prevented by the addition of sacubitrilat [43].
Figure 2.
Neprilysin (NEP) degrades glucagon into metabolites (Gcg1-21, Gcg1-19, Gcg1-18, Gcg5-18, and Gcg1-13) that do not activate the glucagon receptor (GCGR). Matrix-assisted laser desorption/ionization time of flight identified m/zs in the m/z spectra 1500 to 4500 after recombinant A, human, and B, mouse NEP (10 ng) mixed with glucagon (1 µg) after 15, 30, and 60 minutes’ incubation, and after 60 minutes’ incubation with glucagon, NEP, and the NEP inhibitor sacubitrilat (0.5 nmol, 10% dimethyl sulfoxide) (active form of the prodrug, sacubitril). C, GCGR activation (dose-response curve) for native glucagon (Gcg1-29)-induced 3′,5′-cyclic adenosine 5′-monophosphate (cAMP) accumulation in COS-7 cells transiently transfected with human GCGR. D, Dose-response curves for cAMP accumulation with glucagon-degradation products in COS-7 cells transiently transfected with human GCGR (3 technical replicates).
Table 1.
Identified glucagon fragments (m/zs) and corresponding amino acid sequences
| Calculated m/z | Observed m/z | Amino acid sequence | ID | Bond break |
|---|---|---|---|---|
| 3482.8 | 3481.4 | HSQGTFTSDYSKYLDSRRAQDFVQWLMNT | Gcg1-29 | Native glucagon |
| 2462.6 | 2461.3 | HSQGTFTSDYSKYLDSRRAQD | Gcg1-21 | Asp21-Phe22 |
| 2219.4 | 2220.3 | HSQGTFTSDYSKYLDSRRA | Gcg1-19 | Ala19-Gln20 |
| 2148.3 | 2148.3 | HSQGTFTSDYSKYLDSRR | Gcg1-18 | Arg18-Ala19 |
| 1738.9 | 1738.4 | TFTSDYSKYLDSRR | Gcg5-18 | Gly4-Thr5, Arg18-Ala19 |
| 1520.6 | 1520.4 | HSQGTFTSDYSKY | Gcg1-13 | Tyr13-Leu14 |
Abbreviation: ID, identification.
Glucagon Fragments Generated by Neprilysin-Induced Cleavage Do not Activate, Bind to or Antagonize Glucagon Receptor Signaling In Vitro in COS-7 Cells
Having identified the glucagon fragments (Gcg1-21, Gcg1-19, Gcg1-18, Gcg5-18, and Gcg1-13) arising from NEP cleavage of glucagon, we next investigated whether these fragments were capable of modulating GCGR signaling. We therefore assessed their agonistic properties on GCGR activation (evaluated by increases in cAMP accumulation) using COS-7 cells transiently transfected with human or mouse GCGR. Native glucagon, included as a positive control, increased GCGR activity within an expected dynamic range from 0.1 nM to 1 µM with EC50 values of 9.149e–10 M (n = 3) and 1.185e–9 M (n = 3) in COS-7 cells transfected with human (Fig. 2C) or mouse (Supplementary Fig. 3A [41]) GCGR, whereas none of the 5 glucagon fragments had any effect on either the human (Fig. 2D) or mouse (Supplementary Fig. 3B [41]) GCGR. To further evaluate their pharmacological properties, we assessed GCGR binding affinity and antagonistic properties of the glucagon fragments in the same cellular expression system on the human (Supplementary Fig. 4 [41]) and mouse (see Supplementary Fig. 3 [41]) GCGR. Native glucagon displaced [125I]-glucagon radioligand binding in a dose-dependent manner with an IC50 of 8.16e–8 M (n = 3) and 6.44e–8 M (n = 3) in COS-7 cells transfected with human (see Supplementary Fig. 4A [41]) or mouse GCGR (see Supplementary Fig. 3C [41]). None of the glucagon fragments altered the binding of [125I]-glucagon, indicating their inability to bind to human (see Supplementary Fig. 4B [41]) and mouse GCGR (see Supplementary Fig. 3D [41]). The glucagon fragments were also devoid of any antagonistic properties at the human (see Supplementary Fig. 4C [41]) and mouse (see Supplementary Fig. 3E [41]) GCGR, when assessed in the presence of 1 nM native glucagon. Finally, we measured the glucagon fragments using the same immunoassays as were used in the human studies (NH2- and COOH-directed sandwich ELISA and COOH-directed RIA), and found that none of the glucagon fragments derived by NEP cleavage cross-reacted with either of the 2 glucagon immunoassays used to measure glucagon in plasma.
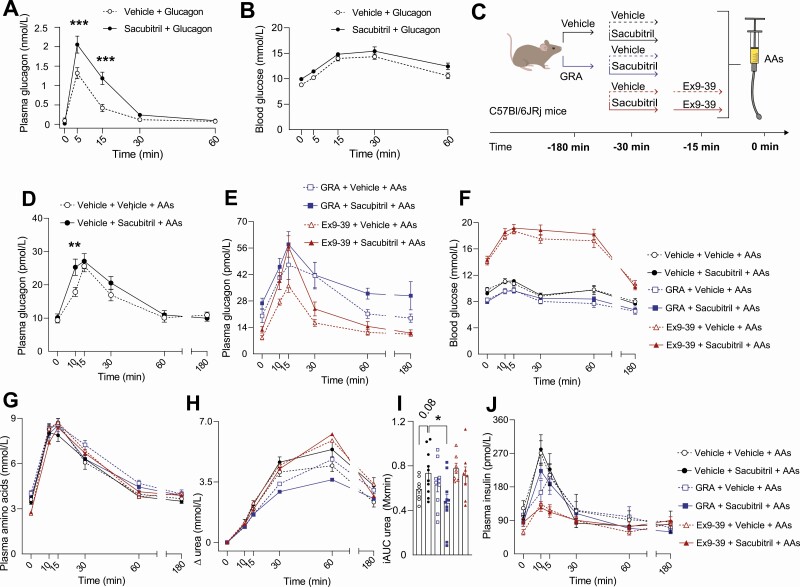
Exogenous and Endogenous Glucagon Concentrations Are Increased in Mice in Response to Pharmacological Neprilysin Inhibition
The effect of NEP inhibition on exogenous glucagon was studied in female C57BL/6JRj mice by administering sacubitril (0.7 nmol/g BW, ~ 60% inhibition of NEP activity in mice; data not shown) 30 minutes prior to a glucagon injection (96 ng/g BW). Sacubitril decreased the degradation of glucagon following exogenous glucagon administration (P = .001) (Fig. 3A) without affecting blood glucose concentrations (Fig. 3B). We investigated the effect of NEP inhibition on endogenous glucagon concentrations by measuring plasma concentrations of glucagon in female C57BL/6JRj mice (and male mice in Supplementary Fig. 5 [41]) after stimulation with oral administration of AAs (AA composition shown in Supplementary Table 1 [41]) (Fig. 3C). Sacubitril increased the glucagon response to AAs by 41% (at time 10 minutes, P = .007) (Fig. 3D). The GLP-1R antagonist (Ex9-39) increased plasma concentrations of AA-stimulated glucagon compared to vehicle treatment (Fig. 3F-G), while coadministration of Ex9-39 and sacubitril increased plasma concentrations of glucagon more when compared to Ex9-39 treatment alone (Fig. 3E). Sacubitril did not, however, increase the glucagon response to AAs in a model of genetic GLP-1R deficiency (GLP-1R–/– mice) (Supplementary Fig. 6 [41]). The augmenting effects of sacubitril on AA-stimulated glucagon levels were apparent only in littermate controls (GLP-1R+/+ mice) (time point 15 minutes, P = .02). Sacubitril increased blood glucose concentrations in response to an AA challenge in GLP-1R–/– mice (P = .03), whereas no significant increase was observed in C57BL/6JRj mice after sacubitril (Fig. 3F) despite increased plasma concentrations of glucagon. Similar experiments were performed in male C57BL/6JRj mice, and comparable results to the findings in female C57BL/6JRj mice were observed (Supplementary Fig. 5A-5C [41]).
Figure 3.
Neprilysin (NEP) inhibition increases plasma concentrations of glucagon in female C57BL/6JRj mice, perturbing amino acid metabolism. Plasma concentrations of A, glucagon, and B, blood glucose concentrations in female C57BL/6JRj mice (n = 10) treated with either vehicle (phosphate-buffered saline + 0.7% dimethyl sulfoxide, open circles) or sacubitril (0.7 nmol/g body weight [BW], black circles) at time point –30 minutes followed by an intraperitoneal injection of glucagon (96 ng/g BW) at time 0 minutes. C, Experimental design used in female C57BL/6JRj mice (n = 7-10) treated with either vehicle (black lines and circles), glucagon receptor antagonist (GRA) (100 mg/kg BW, blue lines and squares), or Ex9-39 (fixed dose of 100 µg in 100 µL, red lines and triangles) with coadministration of vehicle (open symbols) or sacubitril (colored symbols) followed by oral administration of amino acids (AAs, 34 mg) at time 0 minutes. Plasma concentrations of glucagon in mice treated with D, sacubitril or vehicle, and in mice treated with E, GRA, Ex93-39, coadministration of GRA and sacubitril or coadministration of Ex9-39 and sacubitril. F, Blood glucose concentrations, plasma concentrations of G, AAs; H, ∆ urea with I, corresponding incremental areas under the curve (iAUCs); and J, insulin. Data are presented as mean ± SEM and are analyzed by A and D, repeated-measures 2-way analysis of variance, and H, unpaired t tests.
Neprilysin Inhibition May Increase Amino Acid-Stimulated Ureagenesis
Since inhibitors of NEP increase both glucagon and GLP-1 concentrations in humans, we investigated whether the effects of sacubitril on AA catabolism and ureagenesis depend on GCGR and/or GLP-1R signaling. To address this, we treated female C57BL/6JRj mice with a GRA or Ex9-39 and investigated GLP-1R–/– mice (see Supplementary Fig. 6 [41]). As observed previously by our group, GRA increased plasma concentrations of glucagon and AAs while reducing blood glucose levels. NEP inhibition had no effect on plasma concentrations of amino acids when compared to vehicle treatment (Fig. 3G), and coadministration of Ex9-39 and sacubitril as well as coadministration of GRA and sacubitril similarly had no effect on AA concentrations when compared to Ex9-39 treatment and GRA treatment alone (see Fig. 3G). Overall, sacubitril did not appear to reduce plasma concentrations of AAs in this setting, contrary to what we found in healthy young men. In fact, sacubitril unexpectedly increased plasma concentrations of AAs in GLP-1–/– mice as reflected by the iAUC (376 ± 67 vs 255 ± 118, P = .05). As expected, GRA treatment reduced fasting (6 ± 1 vs 8 ± 1 mmol/L, P < .001) and AA-stimulated urea concentrations compared both to vehicle and Ex9-39–treated groups, supporting previous findings showing that GCGR signaling is important for urea production. A trend toward increased AA-stimulated urea levels with sacubitril compared to vehicle treatment was apparent when evaluated by iAUC (737 ± 190 vs 592 ± 97 mmol/L × min, P = .08), and this effect was dependent on GCGR signaling as reflected by the significant difference in urea levels between sacubitril treatment and the combined treatment of sacubitril and GRA (P = .01, Fig. 3H and 3I). Plasma concentrations of urea were similar between groups that were coadministered sacubitril and Ex9-39 compared to sacubitril treatment alone (see Fig. 3I), indicating that the increase in urea with NEP inhibition is independent of GLP-1R signaling. Fasting (143 ± 63 vs 58 ± 21 pmol/L, P = .005) and postprandial insulin concentrations were decreased in Ex9-39–treated groups compared to vehicle treatment, and coadministration of Ex9-39 and sacubitril did not affect plasma concentrations of insulin compared to Ex9-39 treatment alone (Fig. 3J). To assess potential sex differences, we performed an identical experiment in male C57BL/6JRj mice (n = 6) and, similarly to the cohort of female mice, sacubitril increased plasma concentrations of glucagon without altering blood glucose or plasma concentrations of AAs (Supplemental Figure 5 [41]). However, sacubitril did not increase plasma concentrations of urea, contrary to what was observed in female mice. Finally, we repeated the study in female mice (n = 3) that were administered water instead of AAs (Supplementary Fig. 7 [41]) and saw that plasma concentrations of glucagon were increased numerically in groups that were coadministered sacubitril with vehicle, GRA, or Ex9-39 compared to vehicle, GRA, or Ex9-39 alone.
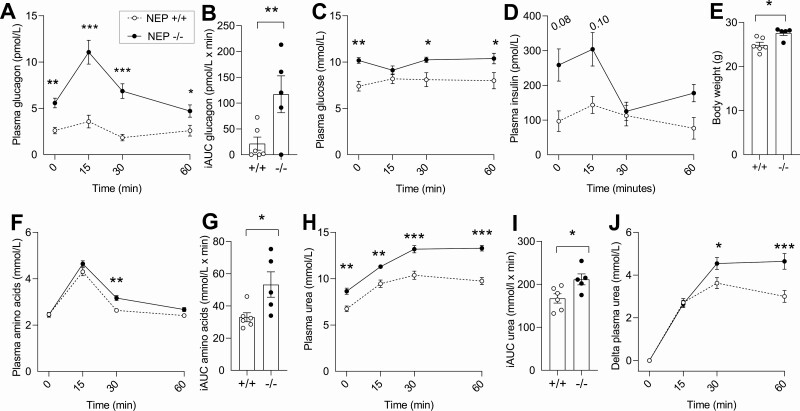
Mice Deficient in Neprilysin Have Increased Plasma Concentrations of Glucagon and Urea
We challenged male NEP–/– mice and controls with AAs by oral gavage (Fig. 4A-4J) to assess the effect of congenital NEP deficiency on endogenous glucagon concentrations. Fasting plasma concentrations of glucagon were 114% higher in NEP–/– mice compared to NEP+/+ mice, and this difference became even more pronounced after an AA challenge (P = .02) (see Fig. 4A and 4B). Fasting blood glucose concentrations were 27% higher in NEP–/– mice compared to NEP+/+ mice (10.2 ± 0.7 vs 7.4 ± 1.3 mmol/L, P = .009), while plasma concentrations of insulin were numerically increased (see Fig. 4C and 4D). NEP–/– mice were heavier than NEP+/+ mice (P = .01) (see Fig. 4E) despite being age-matched. An AA challenge increased plasma concentrations of AAs to a greater extent in NEP–/– mice than in NEP+/+ mice (see Fig. 4F and 4G). Fasting plasma urea concentrations were 22% higher in NEP–/– mice (9 ± 1 vs 7 ± 1 mmol/L, P = .001) compared to NEP+/+ mice, and this difference was further augmented by an AA challenge (P = .03) (see Fig. 4H-4J).
Figure 4.
Neprilysin (NEP)-deficient mice have increased fasting and amino acid-stimulated glucagon and urea concentrations. Plasma concentrations of A, glucagon and B, corresponding incremental areas under the curve (iAUCs); C, blood glucose concentrations, plasma concentrations of D, insulin; E, body weight; plasma concentrations of F, amino acids; and G, corresponding iAUCs, plasma concentrations of H, urea; and I, corresponding iAUCs; and plasma concentrations of J, ∆ urea in male NEP–/– mice (black circles) and NEP+/+ mice (open circles)-challenged amino acids (equivalent to 1% of body weight) at time 0 minutes (n = 5-6). Data are presented as mean ± SEM and are analyzed by A, C, D, F, H, and J, repeated-measures 2-way analysis of variance, and B, E, G and I, unpaired t tests. –/– indicates NEP–/– mice and +/+ indicates NEP+/+ mice.
Discussion
The present study demonstrates that the endopeptidase NEP plays an important role in glucagon metabolism both in humans and mice, and that pharmacological inhibition of NEP induces hyperglucagonemia, with potential effects on AA levels in humans but with negligible effects on glucose levels. The latter may potentially be due to increased GLP-1R signaling, as supported by the increase in postprandial glucose concentrations observed on NEP inhibition in GLP-1R–/– mice as shown here and the finding that NEP also appears to affect plasma concentrations of GLP-1 in humans [5]. Hyperglucagonemia may affect AA catabolism by increasing hepatic AA uptake [44] and ureagenesis [12, 45]. The present data suggest that NEP inhibition may induce the effects on AA metabolism as seen phenotypically with hyperglucagonemia although species and sex differences were observed. The reason for these differences is not clear and warrant further investigation. Given that AA abnormalities are reported in patients with heart failure [46], it may be speculated that sacubitril/valsartan-induced hyperglucagonemia could affect metabolic function through altered AA catabolism.
First, we investigated the effect of NEP inhibition with sacubitril/valsartan on postprandial glucagon concentrations in healthy young men and found increased postprandial glucagon concentrations (identified identically by sandwich ELISA and by a COOH-terminal wrapping RIA), consistent with an earlier experimental study in pigs [11]. These observations align with our present findings by mass spectrometry, demonstrating that glucagon is directly cleaved by NEP, resulting in the formation of glucagon fragments that are truncated in the COOH-terminal and therefore lose their immunoreactivity. We do consider the use of sacubitril/valsartan (as opposed to sacubitril alone, which is not available for use in humans) as a limitation to the study. However, the finding that valsartan alone had no effect on glucagon levels in a subset of individuals (n = 3), together with our in vitro and mouse data indicating that glucagon concentrations are altered in response to changes in NEP activity, leads us to suggest that the valsartan component of the drug is probably not responsible for the observed changes in the plasma concentrations of glucagon.
Sacubitril/valsartan increased plasma concentrations of glucagon in healthy young men and individuals with obesity without affecting glucose concentrations. However, long-term sacubitril/valsartan treatment is reported to improve glycemic control in individuals with obesity and type 2 diabetes [8, 24]. While this may be counterintuitive in view of the data shown here, it is pertinent to recognize that NEP affects a wide range of additional peptide hormones, including ANP, through direct cleavage by NEP, as well as GLP-1 [5, 47] possibly through reduced dipeptidyl peptidase 4 (DPP-4) activity [48] and/or direct cleavage [49]. GLP-1 may affect glucose levels via its well-known insulinotropic effects [50], and ANP may also improve glycemia [51, 52]. The unaltered plasma concentrations of glucose in the cohorts investigated here may, therefore, be due to increased GLP-1 and/or ANP receptor signaling counterbalancing glucagon’s effects on hepatic glucose production. Indeed, we find that sacubitril increases blood glucose concentrations in GLP-1R–/– mice compared to vehicle treatment, supporting the notion that increased GLP-1R signaling in response to NEP inhibition may counteract the effects of glucagon on glucose levels. It has also been shown that glucagon secretion—augmented by AAs—stimulates the β cell via their GLP-1R and thereby lowers glycemia [53]. At any rate, during protein intake, which stimulates both glucagon and insulin secretion, increases in plasma concentrations of glucagon do not necessarily lead to increases in plasma concentrations of glucose [54, 55].
In accordance with our own [12] and others’ studies reporting an acute role for glucagon on AA metabolism [13], the sacubitril/valsartan-induced increase in the postprandial concentrations of glucagon in healthy young men was accompanied by a reduction in the concentration of postprandial AAs. In fact, AA concentrations did appear to correlate with glucagon concentrations (based on AUC values) supporting the relationship between glucagon and AA catabolism. On the other hand, this statistical correlation was lost when removing a single data point (for an individual with a very high glucagon AUC), which likely suggests a power issue regarding this subanalysis. The concentration of some AAs, including those that have been suggested to be involved in the liver–α-cell axis (alanine and proline) [56] and to stimulate glucagon secretion (asparagine) [57], were significantly reduced following sacubitril/valsartan treatment. Reductions, however, were modest and not apparent for all AAs. It should be noted that these analyses were conducted post hoc on samples that had not been stored optimally for these particular analyses, which may have confounded the results, so these data should be interpreted with caution. In line with our findings in healthy young men, fasting AA concentrations were unchanged after 8 weeks sacubitril/valsartan treatment in individuals with obesity and hypertension. A mixed-meal tolerance test was not performed in these individuals before and after the intervention. This is a limitation, as glucagon-stimulated AA metabolism may be dependent on substrate availability, meaning that the metabolic implications of sacubitril/valsartan-induced hyperglucagonemia may become apparent only when substrate availability is high. Further studies therefore, are needed to explore the consequence of any NEP-induced effect on AA catabolism.
To investigate whether the increase in glucagon concentrations following NEP inhibition occurs because of a direct effect to inhibit NEP cleavage of the glucagon molecule or reflects an indirect mechanism (such as increased glucagon secretion or reduced glucagon clearance in tissues), we studied the degrading effects of recombinant NEP on glucagon in vitro using mass spectrometry. Recombinant human and mouse NEP rapidly (within 15 minutes) and extensively cleaved glucagon and generated several fragments. NEP has a strong preference toward hydrophobic AAs with cleavage at the N-terminal side (P1) of the residue [58], and this was the case for 4 out of 6 cleavage sites shown here. NEP also targets neutral AAs [59], and we show that at least one AA in every bond break was either neutral or hydrophobic. Pharmacological analyses showed that the glucagon fragments did not bind, agonize, or antagonize either the human or mouse GCGR in transiently transfected COS-7 cells, indicating that NEP activity exclusively attenuates GCGR signaling by reducing plasma concentrations of native glucagon through direct cleavage. These data, therefore, suggest that hyperglucagonemia caused by NEP inhibition in vivo at least partly results from reduced enzymatic glucagon degradation, although our findings do not exclude reduced glucagon clearance in tissues [60] or that increased glucagon secretion by pancreatic α cells may contribute, as previously suggested [21, 61].
While NEP directly decreases circulating concentrations of its substrates by cleavage [62-64], we found that AA-stimulated glucagon concentrations were not increased with sacubitril in a model of congenitally disrupted GLP-1R signaling. However, acute coadministration of the GLP-1R antagonist Ex9-39 and sacubitril markedly increased AA-stimulated glucagon concentrations compared to Ex9-39 treatment alone. The GLP-1R–/– mouse strain is a global and lifelong genetically modified mouse model, and has likely developed adaptive mechanisms [65]. Other gene products regulated secondarily to GLP-1R signaling may have influenced results. We also propose that chronic hyperglycemia may have inhibited the secretion of glucagon, as shown previously [66-68]. In our cohort of NEP–/– mice, fasting concentrations of glucose were also increased, which is contrary to previous reports from this mouse strain [27, 48]. There was a discrepancy between the effects on glycemia with the NEP inhibitor compared to NEP–/– mice, which is currently unexplained and warrants further investigation. However, given that higher glucagon concentrations did not affect glucose concentrations in our other models of reduced NEP activity, the elevated glucagon concentrations may not fully explain the elevated glucose concentrations in NEP–/– mice. Plasma concentrations of AAs (post oral AA administration) were also increased in NEP–/– mice and whether this was due to reduced AA uptake in extrahepatic tissue, increased proteolysis, or denotes another explanation is unknown. However, NEP inhibition may increase plasma concentrations of cortisol [69, 70], a steroid hormone that during prolonged elevation can lead to increased proteolysis [72], which may partially explain the increased levels of AAs and glucose in NEP–/– mice.
We investigated the effects of sacubitril in C57BL/6JRj mice treated with either vehicle, a GRA, or Ex9-39 to explore the underlying mechanisms for the reduction in postprandial AAs responses seen with NEP inhibition in healthy young men. Mice treated with Ex9-39 were included, as NEP inhibition has been shown to increase insulin secretion in mouse islets in a GLP-1R–dependent manner [72]. Plasma concentrations of urea were measured as an indicator of AA catabolism because we were unable to sample urine in our mouse studies. However, this is also a limitation to the study, as small changes in plasma urea levels may have been missed because of efficient renal urea excretion [73]. Here we show that mice with a global deficiency of NEP have increased plasma concentrations of urea in response to an AA challenge, which we hypothesized to be dependent on GCGR signaling. Sacubitril numerically (P = .08) increased AA-stimulated urea levels (as indicated by iAUC) in female C57BL/6JRj mice. Furthermore, AA-stimulated urea concentrations were reduced in mice that were coadministered GRA and sacubitril compared to sacubitril alone both in males and females, indicating that the effects with NEP inhibition on AA catabolism may depend on GCGR signaling. However, since sacubitril did not significantly increase plasma levels of urea in female and male C57BL/6JRj mice, inhibitors of NEP may increase AA catabolism by GCGR-independent mechanism(s). AA-stimulated urea concentrations (as indicated by iAUC) were similar in mice that were coadministered Ex9-39 and sacubitril compared to sacubitril alone both in male and female mice, indicating that the effects of NEP inhibition on AA catabolism were independent of GLP-1R signaling.
Taken together, the present study demonstrates, by use of several different experimental approaches, that NEP inhibition reduces the degradation of endogenous glucagon in humans and mice because of the inhibition of NEP-mediated glucagon cleavage. In healthy young men, sacubitril/valsartan reduced postprandial AA concentrations, including some glucagonotropic AAs, which may be a result of increased glucagon-stimulated, substrate-dependent ureagenesis, as we found increased AA-stimulated plasma concentrations of urea in mice deficient in NEP. NEP inhibitors are prescribed to patients with heart failure, and the reported metabolic improvements in these patients may be related to increased concentrations of proglucagon-derived peptides, as demonstrated here and by others [5, 11, 47]. Furthermore, our results contribute to the emerging evidence suggesting that glucagon may contribute to the acute clearance of AAs in a postprandial setting.
Acknowledgments
We thank Jesper Lau, Novo Nordisk A/S, Måløv, Denmark, for providing the GCGR antagonist 26-2548; Finn Gustafsson (Department of Cardiology, Rigshospitalet) for expert advice; and Maibritt Baggesen, Department of Biomedical Sciences, University of Copenhagen and Christine Rasmussen, Department of Clinical Biochemistry, Rigshospitalet, for excellent technical assistance in the pharmacological experiments and the biochemical analyses. The graphical abstract and illustrations in Figures 2 and 3, and Supplementary Figures 2, 3, 6, and 7 were created with Biorender.com.
Financial Support: This work was supported by NNF Project support in Endocrinology and Metabolism–Nordic Region (application No. 34250), an NNF Excellence Emerging Investigator Grant—Endocrinology and Metabolism (application No. NNF19OC0055001), Novartis Pharma AG, Basel, Switzerland (grant to G.H.G. and E.E.B.), the National Institutes of Health (grant Nos. DK-098506 to S.Z. and P30 DK-017047 University of Washington Diabetes Research Center), and the US Department of Veterans Affairs. Novo Nordisk Foundation Center for Protein Research is supported financially by the Novo Nordisk Foundation (grant agreement NNF14CC0001).
Clinical Trial Information : ClinicalTrials.gov Nos. NCT03717688 (registered October 24, 2018) and NCT01631864 (registered June 29, 2012).
Author Contributions: S.A.S.K. and N.J.W.A. designed the study. S.A.S.K. and L.H. designed, performed, and interpreted the MALDI-TOF mass spectrometry experiments. S.A.S.K., S.Z., N.E., S.M., M.W.S., J.E.H., H.K., F.R.C., and K.D.G. designed, performed, and interpreted the animal experiments. M.M.R. designed, performed, and interpreted the pharmacological experiments. D.T., P.D.M., P.P., J.P.G., G.H.G., E.B., J.J.H., C.F.D., and N.J.W.A. designed, performed, and interpreted the clinical studies. S.A.S.K. wrote the manuscript draft. All authors revised the manuscript and accepted the final edition.
Glossary
Abbreviations
- AA
amino acid
- ANP
atrial natriuretic peptide
- BW
body weight
- cAMP
3′,5′-cyclic adenosine 5′-monophosphate
- DMSO
dimethyl sulfoxide
- DPP-4
dipeptidyl peptidase 4
- EC50
half-maximal drug concentration
- ELISA
enzyme-linked immunosorbent assay
- GCGR
glucagon receptor
- GLP-1
glucagon-like peptide 1
- GLP-1R
glucagon-like peptide 1 receptor
- GRA
glucagon receptor antagonist
- iAUC
incremental area under the curve
- IC50
half-maximal inhibitory concentration
- MALDI-TOF
matrix-assisted laser desorption/ionization time of flight
- NEP
neprilysin
- PBS
phosphate-buffered saline
- RIA
radioimmunoassay
- tAUC
total area under the curve
Additional Information
Disclosures: S.Z. previously received funding from Novartis that ended in 2019. The remaining authors have nothing to disclose.
Data Availability
The data sets generated are not publicly available but can be made available from the corresponding author on reasonable request. Supplementary figures to this article and a detailed description of “Materials and Methods” can be found online in a digital research repository [41].
References
- 1. Kenny AJ. Regulatory peptide metabolism at cell surfaces: the key role of endopeptidase-24.11. Biomed Biochim Acta. 1986;45(11-12):1503-1513. [PubMed] [Google Scholar]
- 2. Esser N, Zraika S. Neprilysin inhibition: a new therapeutic option for type 2 diabetes? Diabetologia. 2019;62(7):1113-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bayés-Genís A, Barallat J, Galán A, et al. . Soluble neprilysin is predictive of cardiovascular death and heart failure hospitalization in heart failure patients. J Am Coll Cardiol. 2015;65(7):657-665. [DOI] [PubMed] [Google Scholar]
- 4. Dalzell JR, Seed A, Berry C, et al. . Effects of neutral endopeptidase (neprilysin) inhibition on the response to other vasoactive peptides in small human resistance arteries: studies with thiorphan and omapatrilat. Cardiovasc Ther. 2014;32(1):13-18. [DOI] [PubMed] [Google Scholar]
- 5. Wewer Albrechtsen NJ, Mark PD, Terzic D, et al. . Sacubitril/valsartan augments postprandial plasma concentrations of active GLP-1 when combined with sitagliptin in men. J Clin Endocrinol Metab. 2019;104(9):3868-3876. [DOI] [PubMed] [Google Scholar]
- 6. Nougué H, Pezel T, Picard F, et al. . Effects of sacubitril/valsartan on neprilysin targets and the metabolism of natriuretic peptides in chronic heart failure: a mechanistic clinical study. Eur J Heart Fail. 2019;21(5):598-605. [DOI] [PubMed] [Google Scholar]
- 7. McMurray JJ, Packer M, Desai AS, et al. ; PARADIGM-HF Committees and Investigators . Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail. 2013;15(9):1062-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seferovic JP, Claggett B, Seidelmann SB, et al. . Effect of sacubitril/valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: a post-hoc analysis from the PARADIGM-HF trial. Lancet Diabetes Endocrinol. 2017;5(5):333-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hupe-Sodmann K, McGregor GP, Bridenbaugh R, et al. . Characterisation of the processing by human neutral endopeptidase 24.11 of GLP-1(7-36) amide and comparison of the substrate specificity of the enzyme for other glucagon-like peptides. Regul Pept. 1995;58(3):149-156. [DOI] [PubMed] [Google Scholar]
- 10. Kerr MA, Kenny AJ. The purification and specificity of a neutral endopeptidase from rabbit kidney brush border. Biochem J. 1974;137(3):477-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Trebbien R, Klarskov L, Olesen M, Holst JJ, Carr RD, Deacon CF. Neutral endopeptidase 24.11 is important for the degradation of both endogenous and exogenous glucagon in anesthetized pigs. Am J Physiol Endocrinol Metab. 2004;287(3):E431-E438. [DOI] [PubMed] [Google Scholar]
- 12. Winther-Sørensen M, Galsgaard KD, Santos A, et al. . Glucagon acutely regulates hepatic amino acid catabolism and the effect may be disturbed by steatosis. Mol Metab. 2020;42:101080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suppli MP, Bagger JI, Lund A, et al. . Glucagon resistance at the level of amino acid turnover in obese subjects with hepatic steatosis. Diabetes. 2020;69(6):1090-1099. [DOI] [PubMed] [Google Scholar]
- 14. Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest. 2012;122(1):4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Unger RH, Orci L. Physiology and pathophysiology of glucagon. Physiol Rev. 1976;56(4):778-826. [DOI] [PubMed] [Google Scholar]
- 16. Unger RH, Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet. 1975;1(7897):14-16. [DOI] [PubMed] [Google Scholar]
- 17. Müller WA, Faloona GR, Aguilar-Parada E, Unger RH. Abnormal alpha-cell function in diabetes. Response to carbohydrate and protein ingestion. N Engl J Med. 1970;283(3):109-115. [DOI] [PubMed] [Google Scholar]
- 18. Müller WA, Faloona GR, Unger RH. The effect of alanine on glucagon secretion. J Clin Invest. 1971;50(10):2215-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Unger RH, Aguilar-Parada E, Müller WA, Eisentraut AM. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest. 1970;49(4):837-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kazda CM, Ding Y, Kelly RP, et al. . Evaluation of efficacy and safety of the glucagon receptor antagonist LY2409021 in patients with type 2 diabetes: 12- and 24-week phase 2 studies. Diabetes Care. 2016;39(7):1241-1249. [DOI] [PubMed] [Google Scholar]
- 21. Longuet C, Robledo AM, Dean ED, et al. . Liver-specific disruption of the murine glucagon receptor produces α-cell hyperplasia: evidence for a circulating α-cell growth factor. Diabetes. 2013;62(4):1196-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Solloway MJ, Madjidi A, Gu C, et al. . Glucagon couples hepatic amino acid catabolism to mTOR-dependent regulation of α-cell mass. Cell Rep. 2015;12(3):495-510. [DOI] [PubMed] [Google Scholar]
- 23. Galsgaard KD, Winther-Sørensen M, Ørskov C, et al. . Disruption of glucagon receptor signaling causes hyperaminoacidemia exposing a possible liver-alpha-cell axis. Am J Physiol Endocrinol Metab. 2018;314(1):E93-E103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jordan J, Stinkens R, Jax T, et al. . Improved insulin sensitivity with angiotensin receptor neprilysin inhibition in individuals with obesity and hypertension. Clin Pharmacol Ther. 2017;101(2):254-263. [DOI] [PubMed] [Google Scholar]
- 25. Balk-Møller E, Agerlin Windeløv J, Svendsen B, et al. . Glucagon-like peptide-1 and atrial natriuretic peptide in a female mouse model of obstructive pulmonary disease. J Endocr Soc. 2019;4(1):bvz034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lu B, Gerard NP, Kolakowski LF Jr, et al. . Neutral endopeptidase modulation of septic shock. J Exp Med. 1995;181(6):2271-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zraika S, Koh DS, Barrow BM, Lu B, Kahn SE, Andrikopoulos S. Neprilysin deficiency protects against fat-induced insulin secretory dysfunction by maintaining calcium influx. Diabetes. 2013;62(5):1593-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kodra JT, Jørgensen AS, Andersen B, et al. . Novel glucagon receptor antagonists with improved selectivity over the glucose-dependent insulinotropic polypeptide receptor. J Med Chem. 2008;51(17):5387-5396. [DOI] [PubMed] [Google Scholar]
- 29. Steenberg VR, Jensen SM, Pedersen J, et al. . Acute disruption of glucagon secretion or action does not improve glucose tolerance in an insulin-deficient mouse model of diabetes. Diabetologia. 2016;59(2):363-370. [DOI] [PubMed] [Google Scholar]
- 30. Galsgaard KD, Winther-Sørensen M, Pedersen J, et al. . Glucose and amino acid metabolism in mice depend mutually on glucagon and insulin receptor signaling. Am J Physiol Endocrinol Metab. 2019;316(4):E660-E673. [DOI] [PubMed] [Google Scholar]
- 31. Ksander GM, Ghai RD, deJesus R, et al. . Dicarboxylic acid dipeptide neutral endopeptidase inhibitors. J Med Chem. 1995;38(10):1689-1700. [DOI] [PubMed] [Google Scholar]
- 32. Bourne A, Kenny AJ. The hydrolysis of brain and atrial natriuretic peptides by porcine choroid plexus is attributable to endopeptidase-24.11. Biochem J. 1990;271(2):381-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stephenson SL, Kenny AJ. The hydrolysis of α-human atrial natriuretic peptide by pig kidney microvillar membranes is initiated by endopeptidase-24.11. Biochem J. 1987;243(1):183-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gabe MBN, Sparre-Ulrich AH, Pedersen MF, et al. . Human GIP(3-30)NH2 inhibits G protein-dependent as well as G protein-independent signaling and is selective for the GIP receptor with high-affinity binding to primate but not rodent GIP receptors. Biochem Pharmacol. 2018;150:97-107. [DOI] [PubMed] [Google Scholar]
- 35. Kissow H, Hartmann B, Holst JJ, et al. . Glucagon-like peptide-1 (GLP-1) receptor agonism or DPP-4 inhibition does not accelerate neoplasia in carcinogen treated mice. Regul Pept. 2012;179(1-3):91-100. [DOI] [PubMed] [Google Scholar]
- 36. Wewer Albrechtsen NJ, Kuhre RE, Windeløv JA, et al. . Dynamics of glucagon secretion in mice and rats revealed using a validated sandwich ELISA for small sample volumes. Am J Physiol Endocrinol Metab. 2016;311(2):E302-E309. [DOI] [PubMed] [Google Scholar]
- 37. Orskov C, Jeppesen J, Madsbad S, Holst JJ. Proglucagon products in plasma of noninsulin-dependent diabetics and nondiabetic controls in the fasting state and after oral glucose and intravenous arginine. J Clin Invest. 1991;87(2):415-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wewer Albrechtsen NJ, Hartmann B, Veedfald S, et al. . Hyperglucagonaemia analysed by glucagon sandwich ELISA: nonspecific interference or truly elevated levels? Diabetologia. 2014;57(9):1919-1926. [DOI] [PubMed] [Google Scholar]
- 39. Smart KF, Aggio RB, Van Houtte JR, Villas-Bôas SG. Analytical platform for metabolome analysis of microbial cells using methyl chloroformate derivatization followed by gas chromatography-mass spectrometry. Nat Protoc. 2010;5(10):1709-1729. [DOI] [PubMed] [Google Scholar]
- 40. Johnsen LG, Skou PB, Khakimov B, Bro R. Gas chromatography–mass spectrometry data processing made easy. J Chromatogr A. 2017;1503:57-64. [DOI] [PubMed] [Google Scholar]
- 41. Kjeldsen SAS, Hansen LH, Esser N, et al. . Neprilysin inhibition increases glucagon levels in humans and mice with potential effects on amino acid metabolism, supplementary file. Uploaded April 17, 2021. doi: 10.6084/m9.figshare.13607534 [DOI]
- 42. Vanneste Y, Michel A, Dimaline R, Najdovski T, Deschodt-Lanckman M. Hydrolysis of α-human atrial natriuretic peptide in vitro by human kidney membranes and purified endopeptidase-24.11. Evidence for a novel cleavage site. Biochem J. 1988;254(2):531-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hansen LH, Madsen TD, Goth CK, et al. . Discovery of O-glycans on atrial natriuretic peptide (ANP) that affect both its proteolytic degradation and potency at its cognate receptor. J Biol Chem. 2019;294(34):12567-12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim J, Okamoto H, Huang Z, et al. . Amino acid transporter Slc38a5 controls glucagon receptor inhibition-induced pancreatic α cell hyperplasia in mice. Cell Metab. 2017;25(6):1348-1361.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Battezzati A, Simonson DC, Luzi L, Matthews DE. Glucagon increases glutamine uptake without affecting glutamine release in humans. Metabolism. 1998;47(6):713-723. [DOI] [PubMed] [Google Scholar]
- 46. Aquilani R, La Rovere MT, Corbellini D, et al. . Plasma amino acid abnormalities in chronic heart failure. mechanisms, potential risks and targets in human myocardium metabolism. Nutrients. 2017;9(11):1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Plamboeck A, Holst JJ, Carr RD, Deacon CF. Neutral endopeptidase 24.11 and dipeptidyl peptidase IV are both mediators of the degradation of glucagon-like peptide 1 in the anaesthetised pig. Diabetologia. 2005;48(9):1882-1890. [DOI] [PubMed] [Google Scholar]
- 48. Willard JR, Barrow BM, Zraika S. Improved glycaemia in high-fat-fed neprilysin-deficient mice is associated with reduced DPP-4 activity and increased active GLP-1 levels. Diabetologia. 2017;60(4):701-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Windeløv JA, Wewer Albrechtsen NJ, Kuhre RE, et al. . Why is it so difficult to measure glucagon-like peptide-1 in a mouse? Diabetologia. 2017;60(10):2066-2075. [DOI] [PubMed] [Google Scholar]
- 50. Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409-1439. [DOI] [PubMed] [Google Scholar]
- 51. Uehlinger DE, Weidmann P, Gnädinger MP, et al. . Increase in circulating insulin induced by atrial natriuretic peptide in normal humans. J Cardiovasc Pharmacol. 1986;8(6):1122-1129. [DOI] [PubMed] [Google Scholar]
- 52. Coué M, Barquissau V, Morigny P, et al. . Natriuretic peptides promote glucose uptake in a cGMP-dependent manner in human adipocytes. Sci Rep. 2018;8(1):1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. El K, Capozzi ME, Campbell JE. Repositioning the alpha cell in postprandial metabolism. Endocrinology. 2020;161(11):bqaa169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Capozzi ME, Wait JB, Koech J, et al. . Glucagon lowers glycemia when β-cells are active. JCI Insight. 2019;5(16):e129954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ang T, Bruce CR, Kowalski GM. Postprandial aminogenic insulin and glucagon secretion can stimulate glucose flux in humans. Diabetes. 2019;68(5):939-946. [DOI] [PubMed] [Google Scholar]
- 56. Galsgaard KD, Jepsen SL, Kjeldsen SAS, Pedersen J, Wewer Albrechtsen NJ, Holst JJ. Alanine, arginine, cysteine, and proline, but not glutamine, are substrates for, and acute mediators of, the liver-α-cell axis in female mice. Am J Physiol Endocrinol Metab. 2020;318(6):E920-E929. [DOI] [PubMed] [Google Scholar]
- 57. Rocha DM, Faloona GR, Unger RH. Glucagon-stimulating activity of 20 amino acids in dogs. J Clin Invest. 1972;51(9):2346-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pozsgay M, Michaud C, Liebman M, Orlowski M. Substrate and inhibitor studies of thermolysin-like neutral metalloendopeptidase from kidney membrane fractions. Comparison with bacterial thermolysin. Biochemistry. 1986;25(6):1292-1299. [DOI] [PubMed] [Google Scholar]
- 59. Feracci H, Maroux S. Rabbit intestinal aminopeptidase N. Purification and molecular properties. Biochim Biophys Acta. 1980;599(2):448-463. [DOI] [PubMed] [Google Scholar]
- 60. Zhou A, Pacini G, Ahrén B, D’Argenio DZ. Glucagon clearance is regulated by nutritional state: evidence from experimental studies in mice. Diabetologia. 2014;57(4):801-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gelling RW, Du XQ, Dichmann DS, et al. . Lower blood glucose, hyperglucagonemia, and pancreatic α cell hyperplasia in glucagon receptor knockout mice. Proc Natl Acad Sci U S A. 2003;100(3):1438-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bayes-Genis A, Barallat J, Richards AM. A test in context: neprilysin: function, inhibition, and biomarker. J Am Coll Cardiol. 2016;68(6):639-653. [DOI] [PubMed] [Google Scholar]
- 63. Spillantini MG, Sicuteri F, Salmon S, Malfroy B. Characterization of endopeptidase 3.4.24.11 (“enkephalinase”) activity in human plasma and cerebrospinal fluid. Biochem Pharmacol. 1990;39(8):1353-1356. [DOI] [PubMed] [Google Scholar]
- 64. Yandle T, Richards M, Smith M, Charles C, Livesey J, Espiner E. Assay of endopeptidase-24.11 activity in plasma applied to in vivo studies of endopeptidase inhibitors. Clin Chem. 1992;38(9):1785-1791. [PubMed] [Google Scholar]
- 65. Snider P, Conway SJ. Probing human cardiovascular congenital disease using transgenic mouse models. Prog Mol Biol Transl Sci. 2011;100:83-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gromada J, Franklin I, Wollheim CB. α-Cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev. 2007;28(1):84-116. [DOI] [PubMed] [Google Scholar]
- 67. Ravier MA, Rutter GA. Glucose or insulin, but not zinc ions, inhibit glucagon secretion from mouse pancreatic α-cells. Diabetes. 2005;54(6):1789-1797. [DOI] [PubMed] [Google Scholar]
- 68. Hahn HJ, Ziegler M. Investigations on isolated islets of langerhans in vitro. 16.Modification of the glucose-dependent inhibition of glucagon secretion. Biochim Biophys Acta. 1977;499(3):362-372. [DOI] [PubMed] [Google Scholar]
- 69. Lainchbury JG, Richards AM, Nicholls MG, Espiner EA, Yandle TG. Brain natriuretic peptide and neutral endopeptidase inhibition in left ventricular impairment. J Clin Endocrinol Metab. 1999;84(2):723-729. [DOI] [PubMed] [Google Scholar]
- 70. Charles CJ, Espiner EA, Richards AM, Sybertz EJ. Endopeptidase inhibition in angiotensin-induced hypertension. Effect of SCH 39370 in sheep. Hypertension. 1995;26(1):89-94. [DOI] [PubMed] [Google Scholar]
- 71. Simmons PS, Miles JM, Gerich JE, Haymond MW. Increased proteolysis. An effect of increases in plasma cortisol within the physiologic range. J Clin Invest. 1984;73(2):412-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Esser N, Barrow BM, Choung E, Shen NJ, Zraika S. Neprilysin inhibition in mouse islets enhances insulin secretion in a GLP-1 receptor dependent manner. Islets. 2018;10(5):175-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Weiner ID, Mitch WE, Sands JM. Urea and ammonia metabolism and the control of renal nitrogen excretion. Clin J Am Soc Nephrol. 2015;10(8):1444-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated are not publicly available but can be made available from the corresponding author on reasonable request. Supplementary figures to this article and a detailed description of “Materials and Methods” can be found online in a digital research repository [41].