Abstract
The relationship between testosterone and premature mortality has caused recent controversy. While previous studies have demonstrated mixed results, this is partly because of variable patient populations, different testosterone thresholds, and the use of antiquated techniques to measure serum testosterone. Using the National Health and Nutrition Examination Survey we analyzed a cohort representative of men in the USA to explore the relationship between serum testosterone and premature mortality using contemporary guidelines and testosterone measurements. We found that men with low testosterone (<300 ng/dl) were at higher risk (odds ratio 2.07, 95% confidence interval 1.30–3.32; p < 0.01) of premature death compared to men with normal testosterone. Furthermore, men with low testosterone were also more likely to have treatable comorbid conditions that were independently predictive of premature mortality. Both testosterone and these comorbid conditions are also modulated by lifestyle modifications, rendering this an important therapeutic approach in men with either or both conditions.
Patient summary
We explored the relationship between testosterone levels and premature death in a large US population. We found that low testosterone is associated with both premature death and related disease processes such as obesity, both of which can be initially treated with diet and exercise.
Keywords: Testosterone, Hypogonadism, Low testosterone, National Health and Nutrition Examination Survey, Premature mortality
The association between low testosterone (LT) and the risk of premature mortality is unclear but has significant implications for screening and treatment guidelines. Prior studies examining this association have found conflicting results, possibly owing to differences in testosterone (T) assays and LT threshold values, whereas others were limited by lack of generalizability [1], [2], [3], [4]. To address these limitations, we used a contemporary guideline-based definition of LT to examine the association between LT and mortality in a US nationally representative cohort of noninstitutionalized men in whom serum T was previously measured without clinical indication [5].
We analyzed data from the National Health and Nutrition Examination Survey (NHANES) that were linked to the National Death Index (NDI) through December 31, 2015 using a probabilistic matching algorithm [6]. In brief, the NDI, a program of the US National Center for Health Statistics, contains information on all death records in the USA after 1979, submitted as statewide data approximately 11 mo after the end of the calendar year. All NHANES participants provide written consent, as described elsewhere [6].
Our analysis included all men aged 40–79 yr with available morning serum T levels (NHANES cycles 1999–2000, 2003–2004, 2011–2012, and 2013–2014). In the earlier cycles (1999–2004), one-third of all men (selected for an unrelated reason) underwent testosterone testing, whereas all eligible men were tested in later cycles [7]. Men treated with androgen deprivation therapy were excluded (Supplementary Fig. 1). Serum T was measured in eligible men via either immunoassay (1999–2000 and 2003–2004) or isotope dilution liquid chromatography and tandem mass spectrometry (2011–2012 and 2013–2014), as described elsewhere [1], [8], [9], [10].
We estimated the association between LT (<300 ng/dl) and all-cause mortality using Cox proportional-hazard models [5]. To inform future guidelines, we report the age-adjusted association between LT and mortality. Covariates that conferred a risk of premature mortality were also characterized. We used χ2 tests and Student’s t tests, where applicable, to analyze the association between relevant covariates and LT, including the 10-yr atherosclerotic cardiovascular disease (ASCVD) risk score used for cardiac risk stratification, which has been previously validated in NHANES [6]. Sensitivity analysis using the Cox proportional-hazard model controlled for age, body mass index (BMI), disability, history of cancer, and ASCVD was also performed. All statistical analyses were performed using SAS v9.4 (SAS Institute, Cary, NC, USA), with a two-tailed p value <0.05 considered significant.
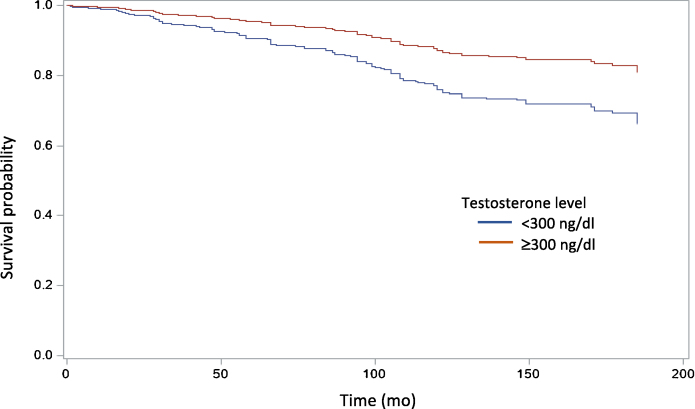
Among 1792 men aged 40–79 yr, 436 (22.11%) had LT and 153 (8.54%) died. Adjusting for age, men with LT were more likely to experience premature death (odds ratio 2.07, 95% confidence interval [CI] 1.30–3.32; p < 0.01; Fig. 1). Additional variables associated with premature mortality included marital status, household income, BMI, ASCVD risk, diabetes (DM), hypertension (HTN), walking disability, history of congestive heart failure (CHF), myocardial infarction (MI), and stroke.
Fig. 1.
Age-adjusted Kaplan-Meier analysis of all-cause mortality for men with and without low testosterone.
Compared to eugonadal men, men with LT were more likely to exhibit obesity, DM, HTN, CHF, MI, walking disability, and a higher ASCVD risk (Table 1). Multivariable analysis demonstrated an independent relationship between LT and premature mortality, with a hazard ratio of 1.66 (95% CI 1.11–2.48; p = 0.01). There were no statistically significant differences in the proportion of specific causes of mortality among deceased men with and without LT (data not shown).
Table 1.
Association of participant characteristics with premature mortality and morning testosterone levels in the National Health and Nutrition Examination Survey.
| Patients, n (%) |
p value | ||
|---|---|---|---|
| T <300 ng/dl | T ≥300 ng/dl | ||
| Marital status | 0.79 | ||
| Unmarried or other | 115 (26.04) | 374 (25.18) | |
| Married or living with a partner | 318 (73.96) | 970 (74.82) | |
| Poverty income ratio | 0.15 | ||
| Below poverty line | 79 (9.75) | 237 (13.04) | |
| At or above poverty line | 319 (90.25) | 1014 (86.96) | |
| Body mass index | <0.01 | ||
| Underweight (<18.5 kg/m2) | 3 (0.5) | 13 (0.78) | |
| Normal (18.5–24.99 kg/m2) | 53 (10.75) | 384 (25.56) | |
| Overweight (25–29.99 kg/m2) | 143 (33.59) | 580 (43.8) | |
| Obese (≥30 kg/m2) | 233 (55.17) | 365 (29.86) | |
| Diabetes | 154 (30.68) | 232 (13.01) | <0.01 |
| Hypertension | 227 (48.01) | 523 (36.37) | <0.01 |
| Walking disability | 96 (18.52) | 198 (10.46) | <0.01 |
| History of congestive heart failure | 34 (7.81) | 51 (3.62) | <0.01 |
| History of heart attack | 42 (10.16) | 79 (5.65) | <0.01 |
| History of stroke | 17 (3.68) | 67 (4) | 0.79 |
| 10-yr atherosclerotic cardiovascular disease risk | 0.01 | ||
| Low risk (<5%) | 140 (35.02) | 497 (41.75) | |
| Borderline risk (5–7.4%) | 35 (9.99) | 163 (14.58) | |
| Intermediate risk (7.5–19.9%) | 136 (34.3) | 442 (30.34) | |
| High risk (≥20%) | 125 (20.68) | 254 (13.32) | |
We found that US men aged 40–79 yr with LT had a higher risk of premature mortality compared to men with normal T. These data are broadly generalizable, address many limitations of previous retrospective studies, and have important clinical implications [2], [3]. Unlike previous work, T measurement in male NHANES participants was not related to symptoms or other clinical indication, thereby limiting sampling bias [2].
The higher risk of premature mortality with LT supports a possible role for screening at-risk individuals even in the absence of hypogonadal symptoms. Our data indicate that men already at risk of premature death (eg, men with obesity, DM, and cardiac morbidity) are also more likely to have LT, which can exacerbate this association, and men with the aforementioned conditions would benefit the most from LT screening. This also demonstrates the importance of guideline-directed care focused on lifestyle changes, which can treat LT and associated comorbidities and ultimately reduce the morbidity and premature mortality associated with LT. The current study must be viewed within the scope of its limitations: it is an observational, time-to-event study; men only had a single T measurement (using 2 different methods); we used a nonharmonized and dichotomized T threshold of <300 ng/dl; and men were selected regardless of symptomatology [11].
Author contributions: Joshua A. Halpern had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Fantus, Halpern, Brannigan, Platz.
Acquisition of data: Fantus, Chang.
Analysis and interpretation of data: Fantus, Chang, Halpern, Brannigan, Platz, Helfand, Bennett.
Drafting of the manuscript: Fantus, Halpern, Platz.
Critical revision of the manuscript for important intellectual content: Halpern, Bennett, Helfand, Brannigan, Platz.
Statistical analysis: Chang, Platz.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Brannigan, Bennett, Helfand.
Other: None.
Financial disclosures: Joshua A. Halpern certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Associate Editor: Silvia Proietti
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.euros.2021.05.008.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Wang C., Catlin D.H., Demers L.M., Starcevic B., Swerdloff R.S. Measurement of total serum testosterone in adult men: comparison of current laboratory methods versus liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab. 2004;89:534–543. doi: 10.1210/jc.2003-031287. [DOI] [PubMed] [Google Scholar]
- 2.Vigen R., O’Donnell C.I., Barón A.E. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829–1836. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- 3.Araujo A.B., Dixon J.M., Suarez E.A., Murad M.H., Guey L.T., Wittert G.A. Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:3007–3019. doi: 10.1210/jc.2011-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgentaler A. Testosterone deficiency and cardiovascular mortality. Asian J Androl. 2015;17:26. doi: 10.4103/1008-682X.143248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulhall J.P., Trost L.W., Brannigan R.E. Evaluation and management of testosterone deficiency: AUA guideline. J Urol. 2018;200:423–432. doi: 10.1016/j.juro.2018.03.115. [DOI] [PubMed] [Google Scholar]
- 6.Loprinzi P.D., Addoh O. Predictive validity of the American College of Cardiology/American Heart Association pooled cohort equations in predicting all-cause and cardiovascular disease-specific mortality in a national prospective cohort study of adults in the United States. Mayo Clin Proc. 2016;91:763–769. doi: 10.1016/j.mayocp.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Nyante S.J., Graubard B.I., Li Y. Trends in sex hormone concentrations in US males: 1988–1991 to 1999–2004. Int J Androl. 2012;35:456–466. doi: 10.1111/j.1365-2605.2011.01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou H., Wang Y., Gatcombe M. Simultaneous measurement of total estradiol and testosterone in human serum by isotope dilution liquid chromatography tandem mass spectrometry. Anal Bioanal Chem. 2017;409:5943–5954. doi: 10.1007/s00216-017-0529-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vesper H.W., Wang Y., Vidal M., Botelho J.C., Caudill S.P. Serum total testosterone concentrations in the US household population from the NHANES 2011–2012 study population. Clin Chem. 2015;61:1495–1504. doi: 10.1373/clinchem.2015.245969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vesper H.W., Bhasin S., Wang C. Interlaboratory comparison study of serum total testosterone corrected measurements performed by mass spectrometry methods. Steroids. 2009;74:498–503. doi: 10.1016/j.steroids.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Travison T.G., Vesper H.W., Orwoll E. Harmonized reference ranges for circulating testosterone levels in men of four cohort studies in the United States and Europe. J Clin Endocrinol Metab. 2017;102:1161–1173. doi: 10.1210/jc.2016-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.