Figure 4.
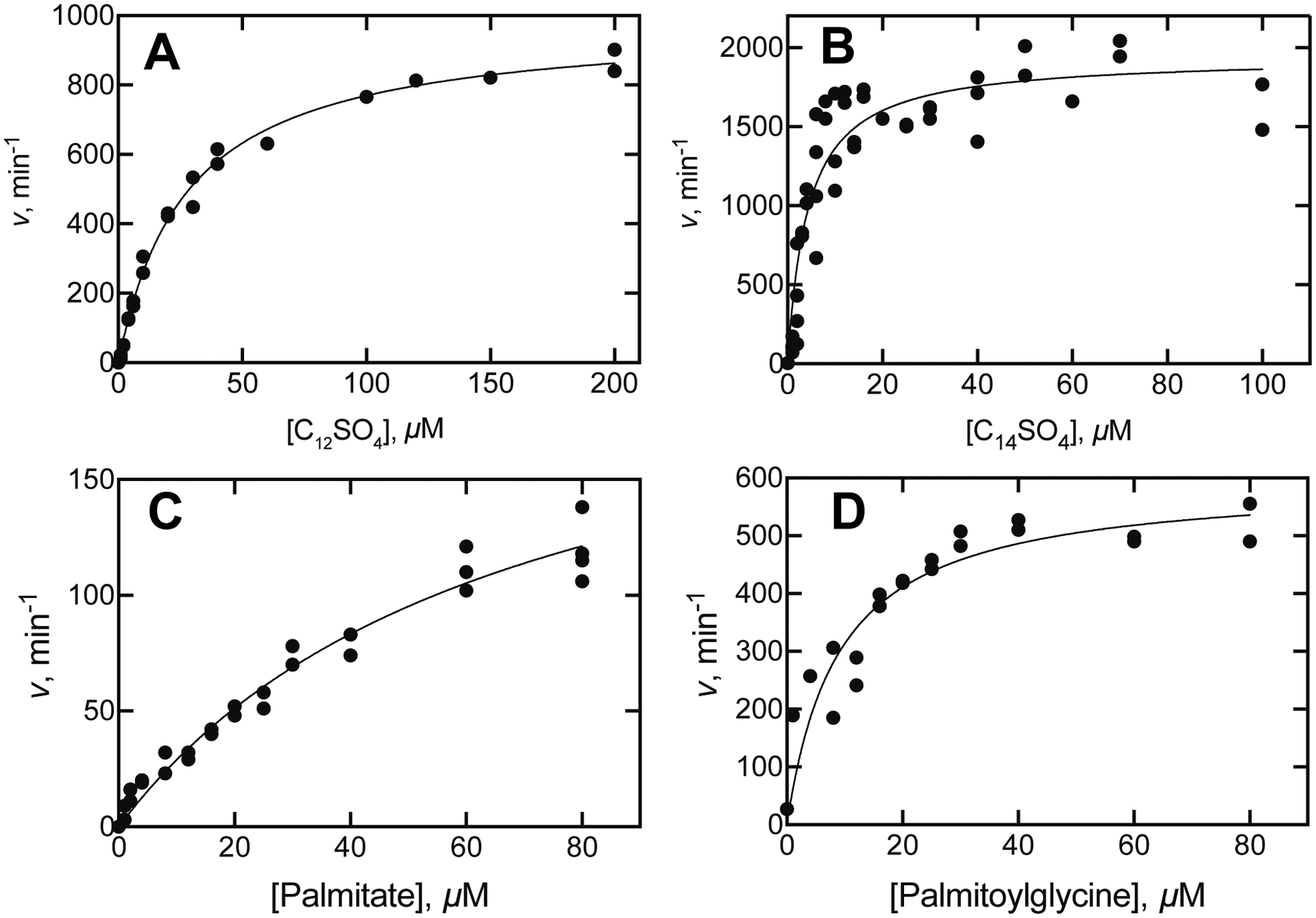
Steady-state kinetics of oxidation of alkyl sulfates and fatty acids by P450BM-3. Measurements were based on rates of oxidation of NADPH and the lines show hyperbolic fits to the data points. A. Dodecyl sulfate (C12SO4): kcat 980 ± 20 min−1, Km 28 ± 1 μM; kcat/Km 36 ± 2 μM−1 min−1 (7.7 × 105 M−1 s−1). B. Tetradecyl sulfate (C14SO4): kcat 1940 min−1, Km 4.2 ± 0.6 μM, kcat/Km 460 ± 60 μM−1 min−1 (3 × 106 M−1 s−1). C. Palmitic acid: kcat 220 ± 20 min−1, Km 67 ± 8 μM, kcat/Km 3.3 ± 0.3 × 105 μM−1 min−1 (5.5 × 104 M−1 s−1). D. N-Palmitoylglycine: kcat 595 ± 40 min−1, Km 9 ± 2 μM, kcat/Km 66 ± 12 μM min−1 (1.1 × 106 M−1s−1).
