Figure 7.
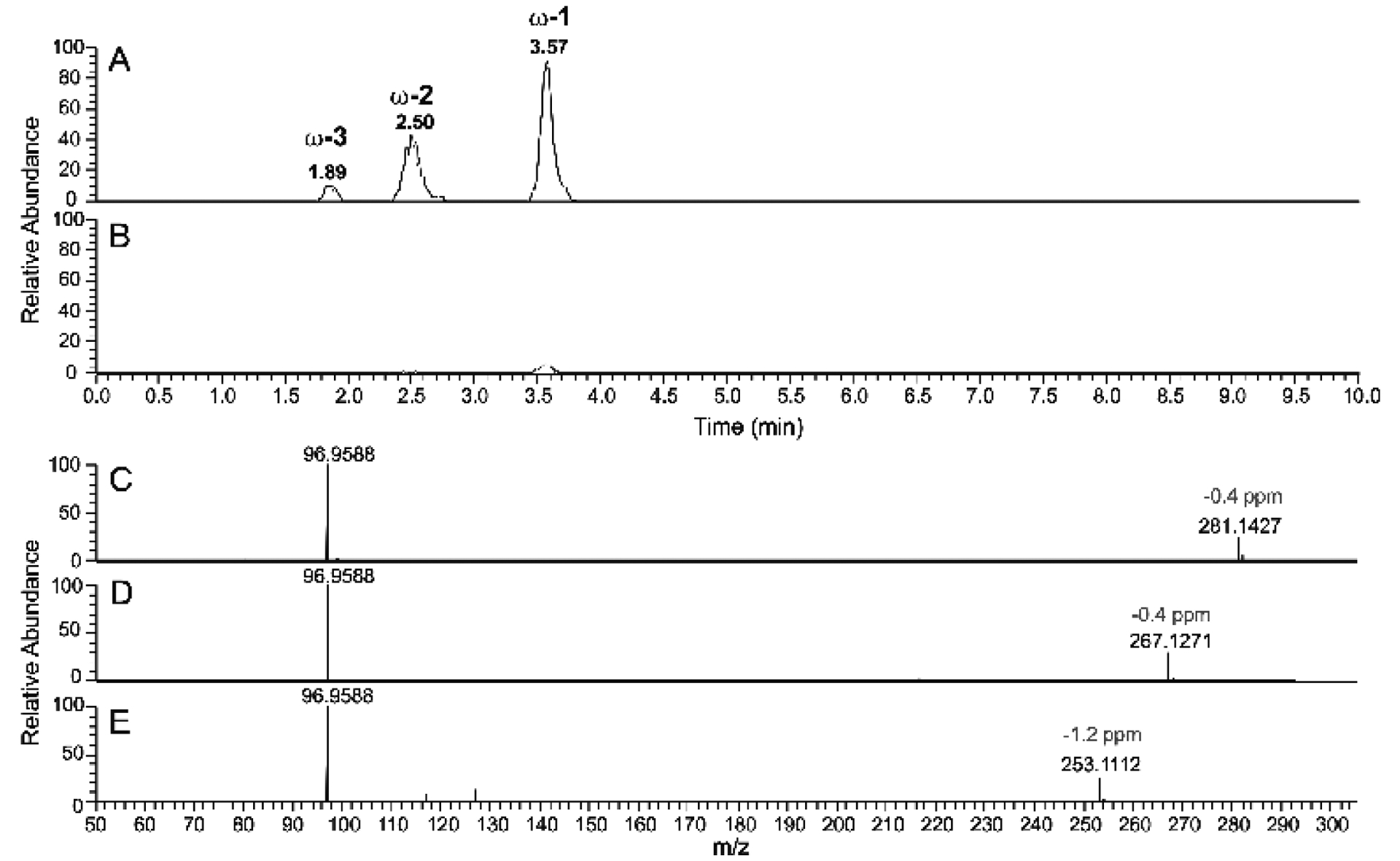
LC-HRMS of P450BM-3 reaction product of tetradecyl sulfate following derivatization to esters and base hydrolysis. A. Tetradecyl sulfate incubated with NADPH and P450BM-3 for 2 minutes. B. Tetradecyl sulfate incubated with NADPH in the absence of P450BM-3. Both reactions were followed by oxidation of alcohol products using Jones reagent,47 Baeyer-Villiger ketone oxidation48 to form esters, and then NaOH treatment to hydrolyze the esters (Scheme 2). Extracted ion chromatograms for m/z 281.1428, 267.1272, and 253.1115 (combined) are shown in Parts A and B, corresponding to the alcohol products detected after base hydrolysis. C-E. HRMS (ESI−) mass fragmentation spectra of the alcohols formed from incubation of tetradecyl sulfate with P450BM-3 and NADPH followed by the two chemical oxidation steps and base hydrolysis: C, fragmentation spectrum of peak eluted at tR 3.57 min peak; D, fragmentation spectrum of peak eluted at tR 2.50 min peak; E, fragmentation spectrum of peak eluted at tR 1.89 min peak.
