Abstract
Introduction
Antibiotic microbial resistance (AMR) is a global health problem. Our aim was to review the resistance of Escherichia (E.coli) to antibiotics at our university hospital over a six-year period and see whether our protocol based antibiotic policy over this time led to any change in the resistance patterns.
Material and methods
Sensitivities of E.coli urine isolates between 2014–2019 (6-years) were sourced from the hospital and general practitioners in the community and collected from the microbiology department. Trends of resistance for amoxicillin, tazocin, cefalexin, ciprofloxacin, co-amoxiclav, gentamicin, nitrofurantoin, trimethoprim, amikacin, and pivmecillinam were examined using the Cochran-Armitage test.
Results
712,004 urine samples tested positive for E. coli. The overall resistance trends for cefalexin, nitrofurantoin and amikacin remained equivocal; increased for ciprofloxacin, co-amoxiclav, gentamicin, and tazocin; and decreased for fosfomycin, pivmecillinam, and trimethoprim.
Conclusions
Despite our protocol based antibiotic policy, although the overall antibiotic resistance remained stable, there was an increasing trend in antibiotic resistance for more commonly used antibiotics including ciprofloxacin, co-amoxiclav, gentamicin, and tazocin reflecting their overall use for prophylaxis and treatment. We plan to continue our policy of reviewing our antibiotic usage and the prescribing protocol with the microbiology department to minimize antibiotic resistance.
Keywords: urinary tract infections, antibiotics, antibiotic microbial resistance, antibiotic resistance, E. Coli, sepsis
INTRODUCTION
Antibiotic microbial resistance (AMR) is a well-documented and growing problem throughout the healthcare systems worldwide. It is estimated to be attributable to at least 300 million excess deaths in the next 35 years [1]. Multi-drug resistant (MDR) organisms such as Extended Spectrum Beta-Lactamase-producing E. coli (ESBL), are difficult to treat, resulting in longer hospital stays and increased mortality [2–4]. Escherichia coli (E. coli) is the causative organism for 80% of community acquired uncomplicated urinary tract infections (UTIs). Therefore, increasing AMR will not only affect hospitals but greatly impact healthcare in the community [5]. The overuse of antibiotics has a well-established association with the development of antibiotic resistance among pathogens [6]. Antibiotic guidelines aim to facilitate antibiotic stewardship by encouraging narrow spectrum prescribing to reduce selection pressures and prevent the development of infections related to broad-spectrum antibiotic use, such as Clostridium difficile [4, 7, 8]. To ensure ongoing efficacy, empirical antimicrobial guidelines require ongoing review of trends in antibiotic susceptibility at local centres. Our aim was to review the resistance of E. coli to antibiotics commonly used at a university hospital over a six-year period to evaluate the effect of our guideline based antibiotic policy on resistance patterns.
MATERIAL AND METHODS
Patient anonymised data from the microbiology department at University Hospital Southampton, UK was collected and collated for the sensitivities of urine cultures testing positive for E. coli over a six-year period between January 2014 and September 2019. Samples were sourced from the hospital and general practitioners in the community. These samples consisted primarily of mid-stream urine culture results from symptomatic patients. Community samples were included as 80% of antibiotic use globally occurs in the community [4]. The susceptibility of E. coli to eleven antibiotics (amikacin, amoxicillin, cefalexin, ciprofloxacin, co-amoxiclav, fosfomycin, gentamicin, nitrofurantoin, pivmecillinam, tazocin and trimethoprim) was reviewed. Urine culture method was primarily based on semi-automated MAST urine culture and any resistant organism like ESBL have further testing based on EUCAST disc sensitivity. The interpretation of sensitivity was based on standard antibiotic sensitivity method, which classifies antimicrobial susceptibility into three groups sensitive, intermediate and resistant. The standard is based on EUCAST Breakpoints. Our laboratory was also accredited with UKAS (United Kingdom Accreditation service) throughout the duration of study, conforming to standard ISO 15189 for medical laboratories.
Trends in resistance were calculated for each antibiotic with Stata using the Cochran-Armitage test for trend carried out at the 5% level, and duplicates were excluded. The degree of resistance trend and its significance was calculated for each antibiotic. Analysis was performed on resistance of urine cultures from all patients sent to the microbiology department.
RESULTS
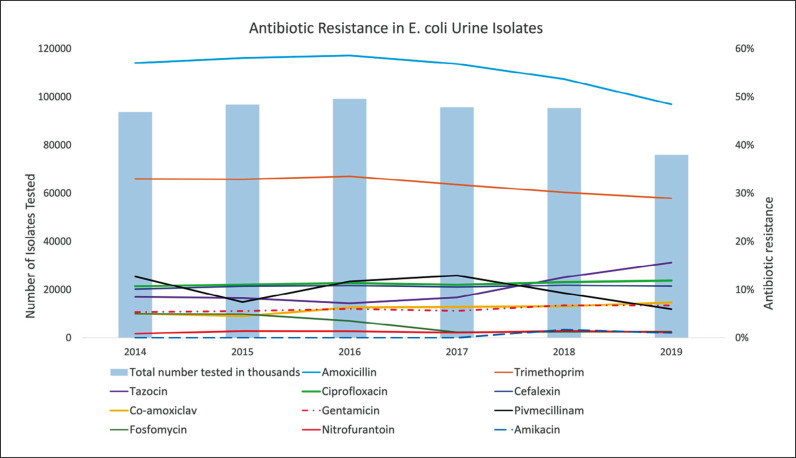
Over six years (2014–2019), a total of 712, 004 urine cultures tested positive for E. coli at our hospital (Table 1). These were sourced from urine samples from hospital inpatients and patients attending their local health centres in the community. Similar to our review of antibiotic resistance between 2007–2011, the antibiotic with the highest frequency of resistance was amoxicillin (48.45%) [9]. The antibiotic with the lowest frequency of resistance was amikacin (1.06%) (Figure 1).
Table 1.
Table displaying the number and percentage resistance of E. coli isolates to antibiotics between 2014–2019 (Res – Resistance)
| Year | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | Trend (p-value) |
|---|---|---|---|---|---|---|---|
| E coli isolates (number of isolates/yr) | 73436 | 75918 | 77853 | 75130 | 75291 | 60385 | |
| Amikacin Res (%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 6 (1.74%) | 7 (1.06%) | Equivocal (p = 0.80) |
| Amoxicillin Res (%) | 6111 (57.02%) | 6455 (58.06%) | 6648 (58.55%) | 6253 (56.84%) | 5901 (53.69%) | 4190 (48.45%) | Decreasing resistance (p = 0.00) |
| Cefalexin Res (%) | 1051 (10.09%) | 1151 (10.68%) | 1196 (10.83%) | 1120 (10.51%) | 1155 (10.89%) | 900 (10.72%) | Equivocal (p = 0.17) |
| Ciprofloxacin Res (%) | 1108 (10.69%) | 1183 (11.04%) | 1253 (11.38%) | 1166 (11.01%) | 1224 (11.56%) | 1000 (11.87%) | Increasing resistance (p = 0.01) |
| Co-amoxiclav Res (%) | 521 (6.03%) | 477 (4.48%) | 689 (6.30%) | 675 (6.38%) | 685 (6.54%) | 618 (7.35%) | Increasing resistance (p = 0.00) |
| Fosfomycin Res (%) | 20 (4.98%) | 23 (4.91%) | 8 (3.51%) | 6 (1.17%) | 7 (1.29%) | 7 (1.30%) | Decreasing resistance (p = 0.00) |
| Gentamicin Res (%) | 551 (5.36%) | 590 (5.55%) | 653 (5.99%) | 589 (5.60%) | 706 (6.72%) | 562 (6.70%) | Increasing resistance (p = 0.00) |
| Nitrofurantoin Res (%) | 88 (0.86%) | 150 (1.42%) | 150 (1.36%) | 113 (1.08%) | 153 (1.46%) | 97 (1.16%) | Equivocal (p = 0.11) |
| Pivmecillinam Res (%) | 52 (12.71%) | 35 (7.45%) | 56 (11.72%) | 64 (12.93%) | 49 (9.28%) | 32 (5.95%) | Decreasing resistance (p = 0.02) |
| Tazocin Res (%) | 60 (8.51%) | 63 (8.26%) | 60 (7.19%) | 64 (8.37%) | 103 (12.55%) | 104 (15.62%) | Increasing resistance (p = 0.00) |
| Trimethoprim Res (%) | 3512 (32.98%) | 3610 (32.89%) | 3779 (33.50%) | 3446 (31.80%) | 3260 (30.18%) | 2483 (28.95%) | Decreasing resistance (p = 0.00) |
Figure 1.
Graph showing the trends in antibiotic resistance in E. coli Urine isolates to eleven antibiotics and the number of isolates tested over six years.
Fosfomycin demonstrated the greatest reduction in antibiotic resistance, falling by 19.22% per year (p <0.001). Amoxicillin, pivmecillinam, and trimethoprim also showed a decrease in antibiotic resistance over the six years (a decreasing trend of 2.74% (p <0.001), 6.25% (p <0.001) and 2.54% (p <0.001) per year respectively). An increasing trend of resistance to ciprofloxacin, co-amoxiclav, and gentamicin was observed (1.84% per year (p <0.01), 10.18% per year (p <0.001), 5.20% per year (p <0.001 respectively). However, the greatest increase in rate of resistance was to tazocin at a rate of 16.56% per year (p <0.001). The overall resistance trends for cefalexin, nitrofurantoin and amikacin remained equivocal.
DISCUSSION
The overuse of antibiotics is associated with increased antibiotic resistance. Between 2000–2010 antibiotic use increased globally by 35% [4]. Antimicrobial guidelines recommend treatments based upon susceptibility patterns to minimise selection pressures and preserve the efficacy of antibiotics at a time when few novel antibiotics are being developed. This study focused on resistance to E. coli as it remains the most common cause of urinary tract infection, contributing greatly to morbidity and health costs affecting all age groups [10]. Due to the prevalence of E. coli, the validity of this study is strengthened by the large number of isolates analysed. We have assumed that the majority of the isolates were taken prior to the commencement of antibiotic treatment as recommended by the guidelines.
Antibiotics with increasing trends in resistance
The European Association of Urology (EAU) guidelines recommend ciprofloxacin as a first line treatment for uncomplicated pyelonephritis [7]. Ciprofloxacin has good prostatic and tissue penetration, covers a broad spectrum of gram-negative bacteria, gram-positive bacteria, and pseudomonas infections and has high bioavailability as both an oral and intravenous preparation [7, 13, 14]. Our findings showed lower rates of ciprofloxacin resistance compared to other English studies [11]. However, an increasing trend in resistance to ciprofloxacin was observed (1.84% per year (p <0.01). This finding corresponds with existing research suggesting a global increase in E. coli resistance to ciprofloxacin, with notably higher rates of resistance observed in developing countries [11, 12]. The observed trend of increasing resistance could be attributed to the overuse of ciprofloxacin [15]. Future studies could compare trends in resistance with prescribing behaviour and adherence to the guidelines by clinicians.
The observed increase in resistance to commonly used antibiotics could be attributed to the treatment of sepsis in addition to the management of urinary tract infections. The trust antibiotic guidelines recommend co-amoxiclav as the first line treatment for sepsis and lower urinary tract infection in pregnant women. The rate of resistance to co-amoxiclav increased by 10.18% per year (p <0.001). Our findings showed a lower prevalence of resistance to co-amoxiclav than the pooled global prevalence found in a recent meta-analysis (7.35% in 2019) [16]. Co-amoxiclav continues to be an effective antibiotic in the management of acute sepsis. However, its value as an efficacious broad-spectrum antibiotic makes it vulnerable to the development of antibiotic resistance. Further efforts should be made to limit or reverse the observed increasing trend in resistance to co-amoxiclav.
A previous study from the University Hospital identified increasing resistance to gentamicin between 2007 to 2011 [9]. Over the six years reviewed in this study the trend in antibiotic resistance to gentamicin continued to increase despite the local antibiotic guidelines (5.2% per year (p <0.001). Overall levels of resistance remained low (6.7% in 2019). This holds clinical relevance as gentamicin is frequently used as a first line treatment for surgical prophylaxis and the management of septic shock. Tazocin is also a valuable resource due to its broad-spectrum coverage. It is recommended in combination with gentamicin for the first line treatment of septic shock. In this study tazocin showed the greatest increase in resistance (16.56% per year, p <0.001). The rise in tazocin resistance reflects its frequent use, emphasising the important role that tazocin plays in everyday clinical practice. With limited antimicrobial resources available as an alternative, ongoing overuse will likely lead to a rise in morbidity and mortality. Close monitoring of this trend will allow us to adjust empirical antibiotic guidelines in an attempt to halt this increasing trend in resistance. To better understand the impact of these findings, future studies could analyse the association between E. coli resistance to antibiotics and patient outcomes.
Antibiotics with decreasing trends in antibiotic resistance
Trimethoprim is often used as an alternative first line antibiotic for the management of uncomplicated cystitis due to its effective prostatic penetration [7]. Across Europe rates of resistance range from 14.6% to 60% with the highest rates of resistance observed amongst young women [12, 17–21]. Our data shows a high level of resistance to trimethoprim (28.95%). In the absence of sensitivities nitrofurantoin may be a better first line treatment for uncomplicated cystitis due to the lower rates of resistance (1.16%). However, our findings suggest that E. coli resistance to trimethoprim is decreasing at a rate of 2.54% per year (p <0.001). This reassuring finding suggests that the implementation of empirical antibiotic guidelines resulted in the reversal of trends in antibiotic resistance, and could over a period of time, increase the susceptibility of E. coli to trimethoprim.
Fosfomycin is also recommended as a first line treatment for women with uncomplicated cystitis [7]. It is preferred by some individuals as it can be taken orally as a one-off dose. Despite being a previously outdated drug, fosfomycin is being explored for clinical consideration in the treatment of complicated UTI due to its low toxicity and unique pharmacokinetics reducing the risk of cross resistance with other antibiotics [22]. Across the six years fosfomycin displayed a decreasing resistance trend of 19.22% per year (p <0.001) with a relatively low rate of resistance (1.3%, 2019). Whilst research is currently insufficient to prompt a change in the guidelines, the use of Fosfomycin as an alternative treatment may alleviate the significant burden placed on other commonly prescribed antimicrobials.
Pivmecillinam and amoxicillin are recommended for the treatment of uncomplicated cystitis in pregnant women [23, 24]. The wide use of amoxicillin for various infections could explain the relatively high rates of resistance noted in the study (48.45%). Reassuringly, both antibiotics displayed decreasing trends of resistance (2.74% per year (p <0.001) and 6.25% per year (p <0.001) respectively). Eliciting the underlying motives behind treatment choices could help to formulate and implement more effective measures to encourage antibiotic stewardship amongst clinicians.
Antibiotics with equivocal trends in resistance
The EAU guidelines recommend nitrofurantoin as the first line treatment for uncomplicated cystitis [7]. It is commonly used due to its cost-effectiveness and high efficiency. Global trends in E. coli resistance to nitrofurantoin suggest a small increase in resistance. However, the incidence of E. coli resistance to nitrofurantoin in Europe remains lower than in South America, Central America, Asia, and the Middle East [14]. A previous study from the university hospital observed a statistically significant decreasing trend in resistance to Nitrofurantoin between 2007–2011 [9]. However, the resistance to nitrofurantoin remained equivocal in this study (p = 0.11). E. coli resistance to nitrofurantoin at our university hospital (1.16%) was lower than resistance levels in other English studies [11]. Promisingly, nitrofurantoin remains a highly effective treatment for uncomplicated cystitis although it should not be used in patients with renal dysfunction.
Cefalexin is recommended in the local policy-based guidelines for uncomplicated cystitis in pregnant women. The data showed that E. coli resistance to cefalexin was relatively high compared to other antibiotics (10.72% in 2019). Whilst the trend observed remained equivocal cefalexin is not the first-line antibiotic due to ongoing high rates of resistance. Amikacin is valuable for the treatment of ESBL E. coli infections. Such organisms are difficult to treat due to multidrug resistance. Our study reassuringly shows that resistance to Amikacin remains low (1.06% in 2019) and trends in resistance remain equivocal. Amikacin continues to be an effective treatment for ESBL UTI.
Antibiotic stewardship
Efforts to disseminate key guidelines pertaining to antibiotic stewardship in the management of UTI were made at the university hospital. Emails were distributed to all members of staff in an attempt to reduce knee jerk antibiotic prescriptions by unnecessarily carrying out urine dipstick testing in asymptomatic patients with long term catheters. Additionally, posters were placed in doctor’s offices to encourage down-grading from broad spectrum antibiotics to a narrow spectrum regimen. The use of smartphone apps such as ‘MicroGuide’ was encouraged during the hospital teaching programme for junior doctors. Evidence suggests that smartphone apps empower users to access local antibiotic guidelines, challenge inappropriate prescribing, and break well-established prescribing behaviours. Additionally, the implementation of guidelines through smartphone apps on the shop floor allowed clinicians to use updated guidelines to adjust prescribing behaviours as resistance trends shift over time [25, 26]. These interventions aimed to encourage antibiotic stewardship through the targeted education of the clinicians prescribing the majority of the antibiotics and the improved accessibility of the trust’s antibiotic guidelines. A hospital antibiogram providing a summary of antimicrobial susceptibility and treatment recommendations based on it would be further helpful [27]. Novel classifications such as usual drug resistance (UDR) and difficult-to-treat resistance (DTR) to clinical practice would help in assessment of the significance of bacterial resistance [27].
Practicalities and areas of future research
Guidelines for empirical antibiotic treatments require ongoing review of antibiotic susceptibility. This study found that overall antibiotic resistance remained stable. Reassuringly, decreasing trends in resistance were seen in four antibiotics, fosfomycin, amoxicillin, pivmecillinam, and trimethoprim. This trend could be attributed to the practice of antibiotic stewardship facilitated by the empirical antibiotic guidelines at the university hospital and community health centres.
The current study did not look into the specific source of where these urine culture samples were obtained from and all urine samples were included irrespective of their source, although majority were mid-stream urine samples. Data on multi-resistant E. coli could not be isolated from the rest of the samples. The increase in Tazocin and Co-amoxiclav resistance may be related to increase in ESBL E. coli. Although the overall antibiotic resistance remained stable, there was an increasing trend in antibiotic resistance for more commonly used antibiotics including tazocin, co-amoxiclav, ciprofloxacin and gentamicin. This could be a reflection of their overall use for prophylaxis and treatment in the acute clinical setting. Increasing trends in antibiotic resistance will require modifications in clinical practice as antibiotic susceptibility decreases in the general population. This poses a great challenge as few novel antibiotics are being developed. Consequent limitations to therapeutic options will result in increased morbidity and mortality. Future research into prescribing behaviours could help to formulate and implement successful interventions to encourage antimicrobial stewardship. As a result the reserve of antibiotic treatments could be maintained to ensure that effective antibiotics are available when we need them [4].
CONCLUSIONS
This findings in this study reinforce the importance of continuous review of resistance trends to update antibiotic guidelines and facilitate antibiotic stewardship amongst prescribing clinicians. This study suggests that ongoing review can prevent or even slow the rise of antibiotic resistance. We plan to continue our policy of reviewing our antibiotic usage and the prescribing protocol with the microbiology department to minimize antimicrobial resistance.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.The Review on Antimicrobial Resistance Chaired by Jim O’Neill Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. https://amr-review.org/sites/default/files/AMR Review Paper - Tackling a crisis for the health and wealth of nations_1.pdf. Accessed September 19, 2020.
- 2.Cantón R, Novais A, Valverde A, et al. Prevalence and spread of extended-spectrum β-lactamase-producing Enterobacteriaceae in Europe. Clin Microbiol Infect. 2008;14(Suppl 1):144–153. doi: 10.1111/j.1469-0691.2007.01850.x. [DOI] [PubMed] [Google Scholar]
- 3.Gajdacs M, Albericio F. Antibiotic resistance: From the bench to patients. Antibiotics (Basel) 2019;8:129. doi: 10.3390/antibiotics8030129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The British Society for Antimicrobial Chemotherapy Antimicrobial stewardship from principles to practice. 2018. https://bsac.org.uk/antimicrobial-stewardship-from-principles-to-practice-e-book/ebook-download/
- 5.Lee DS, Lee S-J, Choe H-S. Community-Acquired Urinary Tract Infection by Escherichia coli in the Era of Antibiotic Resistance. Biomed Res Int. 2018;2018:7656752. doi: 10.1155/2018/7656752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aslam A, Gajdacs M, Zin CS, et al. Evidence of the practice of self-medication with antibiotics among the lay public in low- and middle-income countries: A scoping review. Antibiotics (Basel) 2020;9:597. doi: 10.3390/antibiotics9090597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonkat G, Pickard R, Bartoletti R, et al. EAU Guidelines on Urological Infections. 2019. https://uroweb.org/guideline/urological-infections/
- 8.Bartoletti R, Cai T, Wagenlehner FM, Naber K, Bjerklund Johansen TE. Treatment of Urinary Tract Infections and Antibiotic Stewardship. Eur Urol Suppl. 2016;15:81–87. [Google Scholar]
- 9.Teoh P, Basarab A, Pickering R, Ali A, Hayes M, Somani BK. Changing trends in antibiotic resistance for urinary E. coli infections over five years in a university hospital. J Clin Urol. 2014;7:116–120. [Google Scholar]
- 10.Lagha R, Abdallah F Ben, AL-Sarhan BO, Al-Sodany Y. Antibacterial and Biofilm Inhibitory Activity of Medicinal Plant Essential Oils Against Escherichia coli Isolated from UTI Patients. Molecules. 2019;24:1161. doi: 10.3390/molecules24061161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abernethy J, Guy R, Sheridan EA, et al. Epidemiology of Escherichia coli bacteraemia in England: results of an enhanced sentinel surveillance programme. J Hosp Infect. 2017;95:365–375. doi: 10.1016/j.jhin.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Kot B, Wicha J, Grużewska A, Piechota M, Wolska K, Obrębska M. Virulence factors, biofilm-forming ability, and antimicrobial resistance of urinary Escherichia coli strains isolated from hospitalized patients. Turkish J Med Sci. 2016;46:1908–1914. doi: 10.3906/sag-1508-105. [DOI] [PubMed] [Google Scholar]
- 13.Thai T, Salisbury BH, Zito PM. Ciprofloxacin. StatPearls Publishing; 2020. http://www.ncbi.nlm.nih.gov/pubmed/30571075. Accessed September 17, 2020. [PubMed] [Google Scholar]
- 14.Kot B. Antibiotic Resistance among Uropathogenic Escherichia coli. Polish J Microbiol. 2019;68:403–415. doi: 10.33073/pjm-2019-048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asadi Karam MR, Habibi M, Bouzari S. Urinary tract infection: Pathogenicity, antibiotic resistance and development of effective vaccines against Uropathogenic Escherichia coli. Mol Immunol. 2019;108:56–67. doi: 10.1016/j.molimm.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Bryce A, Hay AD, Lane IF, Thornton HV, Wootton M, Costelloe C. Global prevalence of antibiotic resistance in paediatric urinary tract infections caused by Escherichia coli and association with routine use of antibiotics in primary care: Systematic review and meta-analysis. BMJ. 2016;352:i939. doi: 10.1136/bmj.i939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abduzaimovic A, Aljicevic M, Rebic V, Vranic S, Abduzaimovic K, Sestic S. Antibiotic Resistance in Urinary Isolates of Escherichia coli. Mater Socio Medica. 2016;28:416. doi: 10.5455/msm.2016.28.416-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kresken M, Körber-Irrgang B, Biedenbach DJ, et al. Comparative in vitro activity of oral antimicrobial agents against Enterobacteriaceae from patients with community-acquired urinary tract infections in three European countries. Clin Microbiol Infect. 2016;22:63.e1–63.e5. doi: 10.1016/j.cmi.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 19.Lavigne JP, Bruyère F, Bernard L, et al. Resistance and virulence potential of uropathogenic Escherichia coli strains isolated from patients hospitalized in urology departments: A French prospective multicentre study. J Med Microbiol. 2016;65:530–537. doi: 10.1099/jmm.0.000247. [DOI] [PubMed] [Google Scholar]
- 20.Erb S, Frei R, Tschudin Sutter S, et al. Basic patient characteristics predict antimicrobial resistance in E. coli from urinary tract specimens: a retrospective cohort analysis of 5246 urine samples. Swiss Med Wkly. 2018;148:w14660. doi: 10.4414/smw.2018.14660. [DOI] [PubMed] [Google Scholar]
- 21.Hitzenbichler F, Simon M, Holzmann T, et al. Antibiotic resistance in E. coli isolates from patients with urinary tract infections presenting to the emergency department. Infection. 2018;46:325–331. doi: 10.1007/s15010-018-1117-5. [DOI] [PubMed] [Google Scholar]
- 22.Falagas ME, Kastoris AC, Kapaskelis AM, Karageorgopoulos DE. Fosfomycin for the treatment of multidrug-resistant, including extended-spectrum β-lactamase producing, Enterobacteriaceae infections: a systematic review. Lancet Infect Dis. 2010;10:43–50. doi: 10.1016/S1473-3099(09)70325-1. [DOI] [PubMed] [Google Scholar]
- 23.Graninger W. Pivmecillinam - Therapy of choice for lower urinary tract infection. Int J Antimicrob Agents. 2003;22(Suppl 2):73–78. doi: 10.1016/s0924-8579(03)00235-8. [DOI] [PubMed] [Google Scholar]
- 24.National Institute of Clinical Excellence BNF: British National Formulary.
- 25.Panesar P, Jones A, Aldous A, et al. Attitudes and behaviours to antimicrobial prescribing following introduction of a smartphone App. PLoS One. 2016;11:e0154202. doi: 10.1371/journal.pone.0154202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Microguide Antibiotic resistance, local medical guidance mobile app - MicroGuide No 1. http://www.microguide.eu/. Accessed August 8, 2020.
- 27.Gajdács M, Bátori Z, Ábrók M, Lázár A, Burián K. Characterization of resistance in gram-negative urinary isolates using eexisting and novel indicators of clinical relevancce: A 10-year data analysis. Life (Basel) 10:16. doi: 10.3390/life10020016. 202. [DOI] [PMC free article] [PubMed] [Google Scholar]