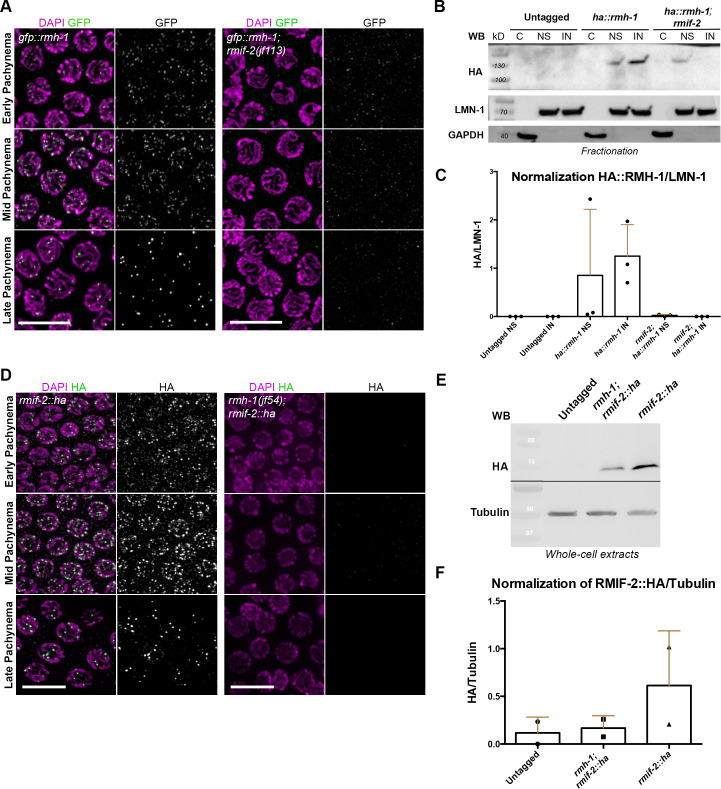
Fig 3. Chromatin loading and abundance of RMIF-2 and RMH-1 proteins are mutually dependent.
(A) Representative images of gfp::rmh-1 and gfp::rmh-1; rmif-2(jf113) pachytene nuclei stained with DAPI (magenta) and GFP (green). GFP::RMH-1 localization to nuclear foci starts in early pachynema, peaks in mid pachynema, and becomes concentrated in six foci in late pachynema. In the rmif-2 mutant background, RMH-1 fails to localize into foci throughout pachynema, except for a very few cytoplasmic foci. Scale bar: 10μm. (B) A protein fractionation shows specific HA::RMH-1 enrichment in the nucleus, which is reduced in the rmif-2 mutant. Equal amounts of protein were loaded for each fraction. C = cytosolic fraction, NS = soluble nuclear fraction, IN = insoluble nuclear fraction. LMN-1 was the loading control for nuclear fractions; GAPDH was the loading control for the cytosolic fraction. (C) Western blot normalization and quantification of nuclear fractionations from untagged WT, ha::rmh-1, and ha::rmh-1; rmif-2 samples. Three biological replicates were used for each sample. (D) Representative images of rmif-2::ha and rmh-1(jf54); rmif-2::ha pachytene nuclei stained with DAPI (magenta) and HA (green). RMIF-2 localization to nuclear foci throughout meiotic prophase starts in early pachytene nuclei, peaks in mid pachynema, and decreases in late pachynema. In the rmh-1 mutant background, RMIF-2 fails to localize to nuclear foci throughout pachynema. Scale bar: 10μm. (E) Western blot analysis of RMIF-2::HA in whole-cell extracts in WT (untagged) and the rmh-1 mutant background. WT worms were used to test the antibody specificity. The predicted size of RMIF-2::HA is 15kD. Tubulin was the loading control. (F) Western blot normalization and quantification of Untagged WT, rmh-1; rmif-2::ha and rmif-2::ha mutants. Two biological replicates were used.