Abstract
Aims
Improving the health status (symptoms, function, and quality of life) of patients with heart failure with reduced ejection fraction (HFrEF) is a primary treatment goal. Angiotensin receptor neprilysin inhibitors (ARNI) improve short‐term health status in clinical practice, but the sustainability of these improvements is unknown.
Methods and results
In CHAMP‐HF, a multicentre observational study of outpatients with HFrEF, patients initiated on ARNI were propensity score matched 1:2 to patients not using ARNI with Cox regression modelling time to ARNI initiation, adjusted for sociodemographic and clinical variables, medical history, medications, and baseline Kansas City Cardiomyopathy Questionnaire (KCCQ) scores. Repeated measures models for the overall KCCQ score and each domain compared the health status trajectories of patients initiated on ARNI vs. not. Among 3930 participants, 746 (19.0%) began ARNI, of whom 576 were matched to 1152 non‐ARNI patients. Prior to matching, participants initiated on ARNI were younger, non‐Hispanic, had lower EFs, more commonly had a history of ventricular arrhythmia, were less likely to be taking an ACEI/ARB, and more likely to be treated with beta‐blockers and mineralocorticoid receptor antagonists. There were no differences after matching. In the matched cohort, participants initiated on ARNI experienced improved health status by 3 months that persisted through 12 months [KCCQ Overall Summary Score (OSS) = 73.4 vs. 70.8; P < 0.001], with the largest benefit observed in the KCCQ Quality of Life domain (68.7 vs. 64.7; P < 0.001). Similar health status benefits were noted through 18 months (KCCQ‐OSS = 73.9 vs. 71.3; P < 0.001). A responder analysis showed that 12 patients would need to be initiated on ARNI for one to experience at least a large improvement (≥10 points) in health status benefit at 12 months.
Conclusions
In outpatient practice, ARNI therapy was associated with improved health status by 3 months and continued to 18 months after initiating therapy.
Keywords: Heart failure reduced ejection fraction, Quality of life, Health status, Angiotensin‐neprilysin inhibitor
Introduction
Improving health status—symptoms, function, and quality of life—in patients with heart failure with reduced ejection fraction (HFrEF) is a primary goal of treatment. 1 In addition to being important to patients and providers, health status is associated with subsequent mortality, hospitalization, and healthcare‐associated cost in HFrEF. 2 , 3 , 4 , 5 The Prospective Comparison of ARNI with ACEI to Determine the Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM‐HF) trial compared sacubitril/valsartan with enalapril in the treatment of patients with HFrEF. 6 Patients taking sacubitril/valsartan not only had improved survival and lower hospitalization rates but also had significantly less deterioration in health status from baseline to 8 months as compared with patients taking enalapril. 6 While other heart failure therapies have also shown reduced mortality in clinical trials, health status improvement with sacubitril/valsartan was a novel finding compared with trials of earlier drugs.
Although there have been a number of randomized controlled trials establishing the efficacy of sacubitril/valsartan, the effectiveness in diverse clinical populations is less clear. Given that the health status improvement was a novel finding and that trial populations, practice settings, and delivery of care differ from real‐world clinical care, supplementing clinical trial data with real‐world experiences can support the effectiveness, as opposed to the efficacy, of new treatments in clinical practice. 7 , 8
In early real‐world evidence from CHAnge the Management of Patients with Heart Failure (CHAMP‐HF), sacubitril/valsartan was associated with improvements in health status as early as 2 months following initiation of the medication 9 ; however, whether these benefits are sustained is unknown. In this study, we aimed to determine whether real‐world early improvements in health status following initiation of sacubitril/valsartan were sustained through 1 year.
Methods
Study design
CHAMP‐HF is a US‐based multicentre, prospective registry of outpatients with HFrEF that serially documented patients' disease‐specific health status and carefully measured changes in patients' medical treatment. 10 Briefly, consecutive patients with chronic HFrEF [left ventricular ejection fraction (LVEF) ≤ 40%] who were treated with ≥1 HFrEF pharmacotherapy were enrolled at 140 outpatient centres across the USA. Patients who were under 18 years of age, currently enrolled or planning to participate in a clinical trial, receiving comfort care measures or hospice care, diagnosed with end‐stage cardiomyopathy with planned heart transplant or left ventricular assist device implantation, and undergoing dialysis were excluded. Study coordinators recruited patients for the registry during the course of routine outpatient visits. To be included in this analysis, patients had to have completed a baseline and at least one follow‐up KCCQ, could not be taking an ARNI prior to enrolment, and could not have a documented contraindication or intolerance to ARNI (Figure 1 ). Clinical data were captured in an electronic case report form at baseline and 1, 3, 6, 12, 18, and 24 months. Patient‐reported data were collected at the same time intervals in person at baseline and in person or by telephone interviews at follow‐up. All study participants provided written informed consent, and each study centre obtained site‐specific institutional review board approval. Novartis Pharmaceuticals Corporation (East Hanover, NJ) sponsored CHAMP‐HF, and Duke Clinical Research Institute (Durham, NC) served as the data analytic centre.
Figure 1.
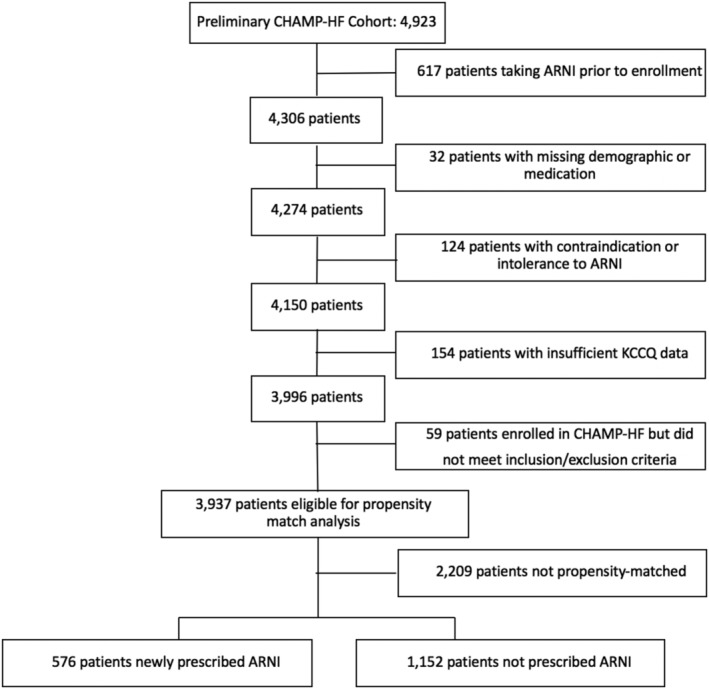
Consort diagram.
Data collection and defining angiotensin receptor neprilysin inhibitors use
Each site collected baseline patient sociodemographic and clinical data, medical and device therapies, and administered the KCCQ at enrolment and 3, 6, 9, and 12 months as well as at 18 and 24 months after enrolment. Patient data were serially collected through in‐person interviews at enrolment and by in‐person or phone interviews during follow‐up. A patient was considered to be a new ARNI start if they began ARNI therapy on or after the enrolment date.
Study outcomes
The primary outcome for this study was patients' health status over time as measured by the KCCQ‐12 Overall Summary (KCCQ‐OS) score. The secondary outcomes were patients' KCCQ domain scores—physical limitation, symptom frequency, quality of life, and social limitation—over time. The KCCQ‐OS and domain scores ranges from 0 to 100 with higher scores associated with fewer symptoms, less functional limitations, and better quality of life. 11 We considered a 5‐point change in score on the KCCQ as a clinically meaningful change in health status based on previously published data, and large and very large clinical changes are associated with changes of 10 and 20 points, respectively. 12 , 13
Statistical analysis
Kaplan–Meier plots were created to describe the unadjusted initiation or discontinuation of ARNI treatment. Because patients who start ARNI may be different from patients who do not, our primary analysis evaluates outcomes within a matched cohort of new ARNI users and non‐ARNI patients. This matching algorithm has been described previously. 9 Briefly, patients initiated on ARNI were matched 1:2 to patients not started on ARNI using time‐dependent propensity scores for ARNI initiation derived from Cox proportional hazard models. These models were used to calculate a propensity score (for each patient and at each value of ARNI start time) that reflects their probability of starting an ARNI at that time. For these models, all predictors (except sociodemographic variables) were allowed to change over time. Models included sociodemographics (age, sex, race, Hispanic ethnicity, household income, and employment status), and clinical factors (body mass index, systolic blood pressure, heart rate, and LVEF), medical history (atrial fibrillation, ventricular tachycardia/ventricular fibrillation, cardiac resynchronization therapy, chronic obstructive pulmonary disease, coronary artery disease, diabetes, depression or anxiety, hypertension, ischaemic heart failure, current smoker, prior HF hospitalizations, and chronic kidney disease), medications (beta‐blocker, mineralocorticoid receptor antagonist, loop diuretic, hydralazine, digoxin, and ivabradine), and baseline KCCQ Symptom Frequency and Quality of Life scores. Continuous variables were assessed for the linearity of their relationship with the outcome using restricted cubic splines, and these terms were included, when necessary, to accommodate nonlinearity. Multi‐level variables were converted to binary variables (white vs. nonwhite, income <$50 K vs. ≥$50 K, and employed full‐time or part‐time vs. not working for any reason).
For covariates in the propensity model, missing values were imputed using single imputation with full conditional specification. Patients had to be either on or off ACEi/ARB for 2 weeks prior to the assigned match day to reduce biasing the differences in KCCQ scores simply due to a change in ACEi/ARB just prior to matching, which led to seven patients being excluded. Covariate balance before and after matching was assessed using standardized differences, with >10% being considered clinically relevant. Median (interquartile ranges) time from matching to last KCCQ score result for both the ARNI and non‐ARNI groups was calculated.
A restricted cubic spline was used to assess the difference in slopes between ARNI and no‐ARNI patients. Outcomes were assessed by five repeated measures regression models: one for the KCCQ‐OS and one for each KCCQ domain score. A patient‐specific random effect was included in each repeated‐measures analysis, as well as a fixed effect for treatment (ARNI) and a fixed effect for time (since match). As patients can report they are limited in their physical function or social function for reasons other than HF, there was some missing data for individual KCCQ domain scores (2.1% for physical limitations and 1.3% for social limitations). If a patient had a missing value for a given KCCQ domain score in the matched set, the mean imputed value for that variable was used. The least square means for the estimated KCCQ scores were calculated from the restricted cubic spline mixed model with 0, 3, 6, 9, 12, and 18 months after matching estimated. Figures displaying the adjusted estimated mean KCCQ score and confidence limits over time are presented.
As mean KCCQ scores represent a population average effect, we also examined the distribution of individual patient change in KCCQ scores to illustrate the proportion of patients with clinically important changes in health status. The proportion of patients (n; %) across categories of KCCQ change [worse to moderate improvement (change <10 points), large improvement (change ≥10 to <20 points), and very large improvement (change ≥20 points)] were calculated.
All estimates were reported using 95% confidence intervals, and a P‐value ≤ 0.05 was considered statistically significant. All analyses were performed using SAS software (version 14.3, SAS Institute, Cary, NC). Analyses were performed independently by the Duke Clinical Research Institute, and the lead author takes responsibility for guiding data analysis and interpretation.
Results
A total of 4923 patients from 140 sites were enrolled in CHAMP‐HF. Over 18 months of follow‐up, 18% (722/3937) of participants were initiated on ARNI (Supporting Information, Figure S1 ). There were 18% (143/792) of patients who were receiving ARNI therapy that discontinued treatment within a year of starting ARNI (Supporting Information, Figure S2 ), but these patients were retained in the analysis to maintain the intention to treat perspective of this study. After excluding patients who were prescribed ARNI prior to enrolment (n = 617), who had a contraindication or intolerance to ARNI (n = 124), with missing demographic or medication data (n = 32), with missing KCCQ data (n = 154), or who did not meet the CHAMP eligibility criteria (n = 59), 3937 patients were eligible for propensity matching, of whom 746 (18.9%) of these were initiated on an ARNI. Prior to matching, patients initiated on ARNI were more likely to be younger, non‐Hispanic, have lower ejection fraction and a history of ventricular arrhythmia, and were less likely to be taking an ACEi or ARB, but were more likely to be on beta‐blocker and mineralocorticoid receptor antagonist (Table 1 ). We were able to match 576 ARNI patients to 1152 patients not initiated on ARNI (Figure 1 ). Matching was well‐balanced for sociodemographic and clinical characteristics, medical history, medications, and baseline KCCQ scores with the exception of the Symptom Frequency and Quality of Life domains (standardized differences = 11.6% and 12.4%, respectively; Table 1 ), resulting in the retention of these scores in the multivariate models. The median time from matching to final KCCQ score was 16.5 months (IQR: 8.6, 22.3) in the ARNI cohort and 15.7 months (IQR: 7.4, 21.8) in the non‐ARNI cohort, and the proportion of deaths were equal between the two groups.
Table 1.
Comparison of baseline patient characteristics before and after matching
| Description | Pre‐match | Post‐match | ||||
|---|---|---|---|---|---|---|
| ARNI | No ARNI | Standardized difference (%) | ARNI | No ARNI | Standardized difference (%) | |
| N | 746 | 3184 | 576 | 1152 | ||
| Sociodemographic | ||||||
| Age | 63.4 (13.0) | 67.3 (12.3) | 30.4 | 64.7 (13.2) | 65.7 (12.5) | 8.2 |
| Female | 29% (217) | 29% (923) | 0.2 | 30% (173) | 27% (310) | 6.9 |
| White race | 75% (559) | 74% (2348) | 2.7 | 76% (437) | 74% (854) | 4.0 |
| Hispanic | 11% (83) | 20% (652) | 25.9 | 12% (67) | 12% (133) | 0.3 |
| Low income (<$25 K) | 38% (285) | 42% (1326) | 7.0 | 37% (214) | 39% (454) | 4.6 |
| Working FT/PT | 26% (195) | 20% (640) | 14.4 | 25% (143) | 24% (279) | 1.4 |
| Clinical measures | ||||||
| BMI | 31.3 (7.3) | 30.1 (7.2) | 15.7 | 31.0 (7.4) | 30.4 (7.4) | 9.1 |
| Systolic BP | 119.9 (17.1) | 122.0 (17.8) | 11.5 | 121.1 (18.1) | 119.8 (17.8) | 7.4 |
| Heart rate | 74.0 (12.7) | 74.0 (12.5) | 0.0 | 74.4 (12.9) | 73.9 (12.7) | 3.8 |
| LVEF | 27.8 (7.8) | 30.2 (7.7) | 30.9 | 30.1 (7.0) | 30.0 (9.0) | 2.0 |
| Medical history | ||||||
| History AF/flutter | 36% (271) | 36% (1150) | 0.4 | 38% (218) | 38% (441) | 0.9 |
| History VT/VF | 25% (183) | 18% (568) | 16.4 | 26% (149) | 24% (276) | 4.4 |
| CRT | 9% (67) | 6% (204) | 9.7 | 11% (61) | 10% (113) | 2.6 |
| Lung disease | 27% (202) | 33% (1036) | 12.0 | 29% (169) | 29% (331) | 1.3 |
| CAD | 61% (458) | 66% (2101) | 9.6 | 63% (361) | 66% (765) | 7.8 |
| Diabetes | 42% (310) | 42% (1342) | 1.2 | 44% (253) | 39% (455) | 9.0 |
| Depression or anxiety | 34% (250) | 36% (1133) | 4.4 | 36% (206) | 36% (420) | 1.4 |
| Hypertension | 83% (617) | 85% (2716) | 7.1 | 83% (480) | 83% (958) | 0.5 |
| Ischaemic HF | 38% (281) | 41% (1303) | 6.7 | 39% (224) | 42% (488) | 7.1 |
| Current smoker | 21% (159) | 20% (633) | 3.5 | 22% (125) | 21% (244) | 1.3 |
| Prior HF hospitalization | 38% (280) | 37% (1172) | 1.5 | 41% (235) | 45% (515) | 7.9 |
| CKD | 19% (143) | 22% (691) | 6.3 | 20% (113) | 21% (240) | 3.0 |
| Medications | ||||||
| ACEi/ARB | 65% (487) | 79% (2529) | 32.0 | 49% (284) | 49% (568) | 0.0 |
| Beta‐blocker | 96% (719) | 90% (2853) | 26.8 | 97% (556) | 97% (1116) | 1.9 |
| MRA | 47% (347) | 32% (1024) | 29.7 | 47% (270) | 44% (505) | 6.1 |
| Loop diuretic | 72% (537) | 63% (1991) | 20.2 | 73% (418) | 70% (812) | 4.6 |
| Hydralazine | 4% (29) | 6% (185) | 9.0 | 5% (27) | 3% (40) | 6.1 |
| Digoxin | 18% (134) | 13% (413) | 13.8 | 17% (99) | 15% (178) | 4.7 |
| Ivabradine | 1% (11) | 1% (32) | 4.2 | 1% (6) | 2% (20) | 5.9 |
| KCCQ scores | ||||||
| Overall score | 64.1 (23.6) | 65.0 (23.6) | 3.5 | 66.9 (23.2) | 69.2 (23.3) | 9.9 |
| Symptom frequency | 68.4 (25.6) | 69.9 (25.0) | 6.1 | 70.6 (25.4) | 73.5 (24.5) | 11.6 |
| QOL | 56.8 (28.6) | 58.5 (28.1) | 6.0 | 59.1 (27.7) | 62.6 (27.3) | 12.4 |
| Social limitations | 65.9 (29.1) | 67.7 (29.1) | 6.3 | 69.8 (28.1) | 72.2 (28.3) | 8.6 |
| Physical limitations | 65.1 (26.0) | 63.8 (27.5) | 5.1 | 67.7 (26.5) | 68.3 (26.8) | 2.0 |
ACEi, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; CAD, coronary artery disease; CKD, chronic kidney disease; CRT, cardiac resynchronization therapy; HF, heart failure; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; QOL, quality of life; VF, ventricular fibrillation; VT, ventricular tachycardia.
Patients initiated on ARNI experienced improved health status compared with patients not on ARNI by 3 months, and these differences persisted to 12 months (P < 0.001; Figure 2 ). Estimated KCCQ‐OS scores at 12 months were 73.4 (95% CI 72.1–74.7) with ARNI vs. 70.8 (95% CI 69.9–71.8) without ARNI. While the largest benefit of ARNI was observed in the KCCQ Quality of Life domain (P < 0.001), there were also statistically significant differences observed in the symptom frequency (P = 0.02) and social limitation (P = 0.007) domains (Figure 3 ). There was not a statistically significant difference in the physical limitation domains between the ARNI and non‐ARNI cohorts (P = 0.16). These health status benefits noted at 12 months remained unchanged through 18 months of follow‐up (Supporting Information, Figures S3 and S4 ).
Figure 2.
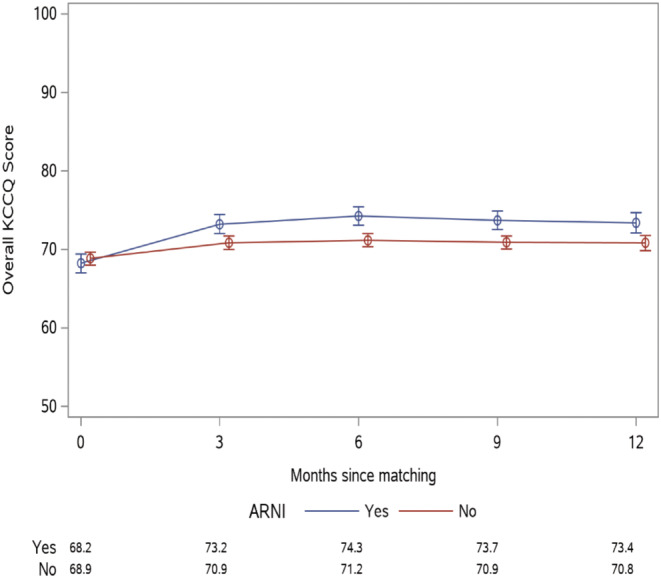
KCCQ Overall Summary Score health status trajectory in patients initiated on ARNI vs. not for 12 months following initiation, P < 0.001.
Figure 3.
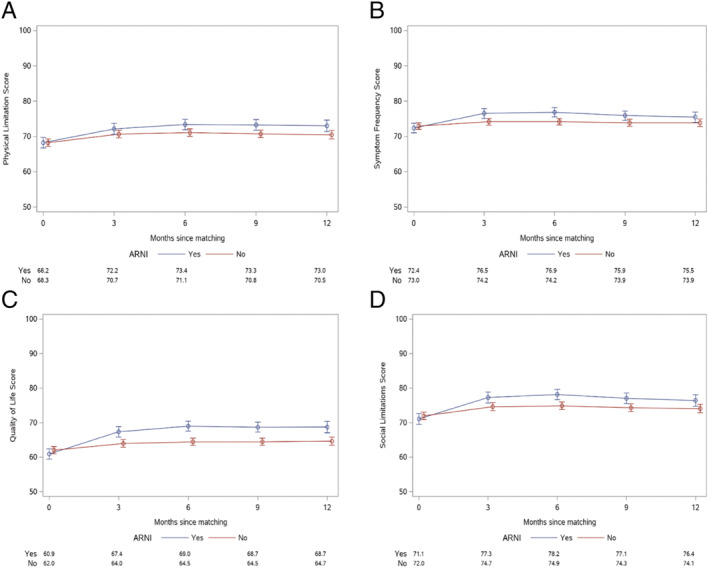
KCCQ domain health status trajectories in patients initiated on ARNI vs. not for 12 months following initiation. (A) Physical limitation score (P = 0.16), (B) symptom frequency score (P = 0.02), (C) quality of life score (P < 0.001), and (D) social limitation score (P = 0.007).
To facilitate the interpretation of the mean differences KCCQ‐OS scores, patients were categorized by the magnitude of health status improvement and the proportion of patients in each category were calculated: those experiencing worse to moderately improved health status (KCCQ scores <10 points), large (10 to <20 points), and very large health status (≥20 points) improvement. Overall, 35.8% of patients initiated on ARNI (vs. 27.5% of patients not initiated on ARNI) had at least a large improvement in their health status over a median of ~10 months, P = 0.001 (Table 2 ) with most of these patients experiencing a very large improvement in their health status (20.3% treated with ARNI vs. 14.7% comparison patients). This corresponds to a number needed to treat of 12, meaning that 12 patients would need to be initiated on ARNI for one to experience at least a large improvement (≥10 points) in health status benefit. A 5‐point responder analysis has been provided in Supporting Information, Appendix S1 .
Table 2.
Change in KCCQ Overall Summary Score
| Change in KCCQ score | ARNI (n = 576) | No ARNI (n = 1152) | P‐value |
|---|---|---|---|
| No change, moderate improvement, or lower scores compared with baseline (<10 points) | 370 (64.2%) | 835 (72.5%) | 0.001 |
| Large improvement (≥10 to <20 points) | 89 (15.5%) | 148 (12.8%) | |
| Very large improvement (≥20 points) | 117 (20.3%) | 169 (14.7%) | |
| Median follow‐up (IQR, in months) | 10.7 (6.9, 11.7) | 10.3 (6.0, 11.6) |
Discussion
In this large, outpatient, observational registry of patients with HFrEF, we found that patients prescribed ARNI experienced both early and sustained improvements in disease‐specific health status as measured by the KCCQ. A similar pattern of improvement was seen for all KCCQ domains, except for the physical limitation domain. While the mean improvement in KCCQ was less than the 5‐point difference considered to be clinically significant, using a population average is not the best method to assess health status as the mean difference does not reflect the experiences of individual patients. We therefore performed a responder analysis that showed that patients initiated on ARNI were significantly more likely to experience a large or very large improvement in health status compared with patients who were not initiated on an ARNI.
Our findings substantially extend the findings from both the clinical trial data from PARADIGM‐HF as well as the findings from a prior CHAMP‐HF analysis. 6 , 9 , 14 , 15 In PARADIGM‐HF, patients randomized to ARNI experienced greater preservation of their health status over 8 months. 14 Not only can health status improvement in the setting of a clinical trial be independent of treatment, due to increased contact with the healthcare system and optimization of medical management, but the baseline KCCQ assessment in PARADIGM‐HF was collected after the run‐in phase, where patients were already on maximal ARNI doses. 16 , 17 It is important, however, to supplement clinical trial data with real‐world ARNI experiences because follow‐up and intensification of medical therapy are variable. To this end, a prior study from the CHAMP‐HF registry showed that initiation of ARNI was associated with early improvements in health status at 2 months. 9 With this current study, we extend these prior findings by showing that early improvements in disease‐specific health status associated with ARNI treatment persist to 18 months in a real‐world setting.
Improving health‐related quality of life is important to both patients and physicians. For patients with heart failure, their quality of life is often considered by them to be as important as their longevity, and for physicians, improving patients' health status is a principal goal of heart failure management. 18 , 19 Despite this, few HF pharmacologic therapies have been shown to improve symptoms, function, and quality of life. While dapagliflozin has been shown in the clinical trial setting to improve quality of life for patients with HFrEF, there is not real‐world evidence available, to date, supporting its effectiveness in improving patients' health status. 20 On the other hand, evidence from CHAMP‐HF has shown that treatment with ARNI results in early and sustained improvement in disease‐specific health status in a real‐world setting. This can help inform patients and clinicians on expectations with initiating ARNI therapy and can be used in patient‐centred decision‐making discussions regarding management.
Although physical limitations improved to a greater extent among the ARNI cohort as compared with the non‐ARNI cohort, this did not reach statistical significance at 12 months. While Khariton et al. showed that there is an early improvement in physical limitations at ~2 months following initiation of ARNI therapy, 4 there are several possible factors that explain our results. First, there is increased heterogeneity of the cohort over the time period from 2 to 12 months. Second, the patients who discontinued ARNI during the study period were retained in the ARNI cohort for the analysis; it is possible that this dissipated the early improvements in physical limitations. Lastly, early benefits seen in functional limitations may stagnate over time. Despite a non‐statistically significant improvement in physical limitations, overall health status was significantly improved in the ARNI cohort over the 12 month time period.
Our findings should be interpreted in the context of the following potential limitations. First as CHAMP‐HF is an observational registry, we cannot exclude bias from unmeasured confounders despite propensity matching. In addition, it is possible the health status benefit observed with ARNI was placebo effect, although the duration and stability of benefit with ARNI makes this less likely. Second, it is worth noting that while patients treated and not treated with ARNI were well matched at baseline, the addition of ARNI includes both an ARB and neprilysin inhibitor. With the addition of both medications, this study cannot distinguish whether patients would have benefited from the addition of an ACEi or ARB alone; nevertheless, the patients initiated on ARNI experienced sustained and significant improvements in health status. Third, the CHAMP‐HF registry includes a broad distribution of outpatient practices, participation of clinical practice sites, physicians, and patients were voluntary, and our results may not be generalizable to patients and clinics that differ from CHAMP‐HF, including those in other countries or in other heart failure settings besides outpatient care. Fourth, due to missingness of some laboratory data, we were unable to match for measures such as B‐type natriuretic peptide levels. Lastly, despite the propensity match including KCCQ scores, the KCCQ scores in the non‐ARNI group were slightly higher at baseline compared with the ARNI group which could have inflated the results of the responder analysis as lower scores have been shown to be stronger predictors of large health status benefits with ARNI. 21
Conclusions
In real‐world clinical practice, the health status benefits associated with ARNI therapy occur within 3 months and persists up to 18 months after initiating therapy. These real‐world data can help support the clinical effectiveness for patient reported outcome measures of ARNI in the outpatient management of patients with HFrEF and can be used to assist physicians in making patient‐centred decisions regarding management.
Conflict of interest
Drs. Thomas and Khariton are supported by the National Heart, Lung, and Blood Institutes of Health Under Award Number T32HL110837; the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Fonarow reports research funding from the National Institutes of Health and serving as a consultant for Abbott; Amgen; AstraZeneca; Bayer; CHF Solutions; Edwards; Janssen; Medtronic; Merck; and Novartis. Dr. Nassif has served as a consultant for Roche Diagnostics; Amgen; and Vifor and has received honorarium from Abbott. Dr. Javed Butler has received research support from the National Institutes of Health and the European Union and serves as a consultant for Amgen; Bayer; Boehringer Ingelheim; Cardiocell; CVRx; Gilead; Janssen; Medtronic; Merck; Novartis; Relypsa; and ZS Pharma. Dr. Laine Thomas reports research funding from Novartis Pharmaceuticals Corporation. Dr. DeVore reports research funding through his institution from the American Heart Association; Amgen; AstraZeneca; Bayer; Intra‐Cellular Therapies; American Regent, Inc; the National Heart, Lung, and Blood Institutes of Health; Novartis; and Patient‐Centered Outcomes Research Institute. He also provides consulting services for Amgen; AstraZeneca; Bayer; CareDx; InnaMed; LivaNova; Mardil Medical; Novartis; Procyrion; scPharmaceuticals; Story Health; and Zoll. He has also received non‐financial support from Abbott for educational activities. Dr. Hernandez reports research funding from American Regent; AstraZeneca; Boehringer Ingelheim; Merck; Novartis; Verily and consulting from Amgen; Bayer; Boston Scientific; Merck; Novartis; and Relypsa. Dr. Albert reports research funding through her institution from Novartis and provides consulting services for Amgen; AstraZeneca; Boston Scientific; and Novartis. Dr. Spertus reports that, relevant to this work, he serves as a consultant for Novartis and owns the copyright to the Kansas City Cardiomyopathy Questionnaire. He also serves as a consultant for Bayer; AstraZeneca; Myokardia; Merck; Amgen; United Healthcare; and Janssen. He has equity in Health Outcomes Sciences and serves on the Board of Directors for Blue Cross‐Blue Shield of Kansas City. The remaining authors have nothing to disclose.
Funding
The CHAMP‐HF registry is funded by Novartis Pharmaceuticals Corporation (East Hanover, NJ, USA).
Supporting information
Figure S1. Rate of patient initiation on ARNI.
Figure S2. Rate of patient discontinuation of ARNI.
Figure S3. KCCQ Overall Summary Score Health Status Trajectory in Patients Initiated on ARNI vs Not Through 18‐months Following Initiation.
Figure S4. KCCQ Domain Health Status Trajectories in Patients Initiated on ARNI vs Not Through 18‐months Following Initiation.
Appendix S1. 5‐point Responder Analysis.
Thomas, M. , Khariton, Y. , Fonarow, G. C. , Arnold, S. V. , Hill, L. , Nassif, M. E. , Chan, P. S. , Butler, J. , Thomas, L. , DeVore, A. D. , Hernandez, A. F. , Albert, N. M. , Patterson, J. H. , Williams, F. B. , and Spertus, J. A. (2021) Association between sacubitril/valsartan initiation and real‐world health status trajectories over 18 months in heart failure with reduced ejection fraction. ESC Heart Failure, 8: 2670–2678. 10.1002/ehf2.13298.
References
- 1. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR. ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013 2013; 128: 1810–1852. [DOI] [PubMed] [Google Scholar]
- 2. Chan PS, Soto G, Jones PG, Nallamothu BK, Zhang Z, Weintraub WS, Spertus JA. Patient health status and costs in heart failure: insights from the eplerenone post‐acute myocardial infarction heart failure efficacy and survival study (EPHESUS). Circulation 2009; 119: 398–407. [DOI] [PubMed] [Google Scholar]
- 3. Pokharel Y, Khariton Y, Tang Y, Nassif ME, Chan PS, Arnold SV, Jones PG, Spertus JA. Association of serial Kansas City Cardiomyopathy Questionnaire assessments with death and hospitalization in patients with heart failure with preserved and reduced ejection fraction: a secondary analysis of 2 randomized clinical trials. JAMA Cardiol 2017; 2: 1315–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heidenreich PA, Spertus JA, Jones PG, Weintraub WS, Rumsfeld JS, Rathore SS, Peterson ED, Masoudi FA, Krumholz HM, Havranek EP, Conard MW. Health status identifies heart failure outpatients at risk for hospitalization or death. J Am Coll Cardiol 2006; 47: 752–756. [DOI] [PubMed] [Google Scholar]
- 5. Soto GE, Jones P, Weintraub WS, Krumholz HM, Spertus JA. Prognostic value of health status in patients with heart failure after acute myocardial infarction. Circulation 2004; 110: 546–551. [DOI] [PubMed] [Google Scholar]
- 6. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 7. Nallamothu BK, Hayward RA, Bates ER. Beyond the randomized clinical trial: the role of effectiveness studies in evaluating cardiovascular therapies. Circulation 2008; 118: 1294–1303. [DOI] [PubMed] [Google Scholar]
- 8. de Lemos JA, Nallamothu BK. The challenges of observational comparative effectiveness research. Circulation 2020; 141: 237–239. [DOI] [PubMed] [Google Scholar]
- 9. Khariton Y, Fonarow GC, Arnold SV, Hellkamp A, Nassif ME, Sharma PP, Butler J, Thomas L, Duffy CI, DeVore AD, Albert NM. Association between sacubitril/valsartan initiation and health status outcomes in heart failure with reduced ejection fraction. JACC Heart Fail 2019; 7: 933–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DeVore AD, Thomas L, Albert NM, Butler J, Hernandez AF, Patterson JH, Spertus JA, Williams FB, Turner SJ, Chan WW, Duffy CI. Change the management of patients with heart failure: rationale and design of the CHAMP‐HF registry. Am Heart J 2017; 189: 177–183. [DOI] [PubMed] [Google Scholar]
- 11. Spertus JA, Jones PG. Development and validation of a short version of the Kansas City Cardiomyopathy Questionnaire. Circ Cardiovasc Qual Outcomes 2015; 8: 469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough PA, Pina I, Tooley J, Weintraub WS, Rumsfeld JS. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J 2005; 150: 707–715. [DOI] [PubMed] [Google Scholar]
- 13. Dreyer RP, Jones PG, Kutty S, Spertus JA. Quantifying clinical change: discrepancies between patients' and providers' perspectives. Qual Life Res 2016; 25: 2213–2220. [DOI] [PubMed] [Google Scholar]
- 14. Lewis EF, Claggett BL, McMurray JJ, Packer M, Lefkowitz MP, Rouleau JL, Liu J, Shi VC, Zile MR, Desai AS, Solomon SD. Health‐related quality of life outcomes in PARADIGM‐HF. Circ Heart Fail 2017; 10. [DOI] [PubMed] [Google Scholar]
- 15. Chandra A, Lewis EF, Claggett BL, Desai AS, Packer M, Zile MR, Swedberg K, Rouleau JL, Shi VC, Lefkowitz MP, Katova T. Effects of sacubitril/valsartan on physical and social activity limitations in patients with heart failure: a secondary analysis of the PARADIGM‐HF trial. JAMA Cardiol 2018; 3: 498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bekelman DB, Plomondon ME, Carey EP, Sullivan MD, Nelson KM, Hattler B, McBryde CF, Lehmann KG, Gianola K, Heidenreich PA, Rumsfeld JS. Primary results of the Patient‐Centered Disease Management (PCDM) for heart failure study: a randomized clinical trial. JAMA Intern Med 2015; 175: 725–732. [DOI] [PubMed] [Google Scholar]
- 17. Lewis EF. Are hospitalizations for heart failure the great equalizer? JACC Heart Fail 2015; 3: 539–541. [DOI] [PubMed] [Google Scholar]
- 18. Kraai IH, Vermeulen KM, Luttik ML, Hoekstra T, Jaarsma T, Hillege HL. Preferences of heart failure patients in daily clinical practice: quality of life or longevity? Eur J Heart Fail 2013; 15: 1113–1121. [DOI] [PubMed] [Google Scholar]
- 19. Lewis EF, Johnson PA, Johnson W, Collins C, Griffin L, Stevenson LW. Preferences for quality of life or survival expressed by patients with heart failure. Heart Lung Transplant 2001; 20: 1016–1024. [DOI] [PubMed] [Google Scholar]
- 20. Kosiborod MN, Jhund PS, Docherty KF, Diez M, Petrie MC, Verma S, Nicolau JC, Merkely B, Kitakaze M, DeMets DL, Inzucchi SE. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: results from the DAPA‐HF trial. Circulation 2020; 141: 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khariton Y, Fonarow GC, Hellkamp A, Thomas L, Nassif ME, Butler J, Duffy CI, Albert NM, Spertus JA. Heterogeneity of health status treatment response with sacubitril/valsartan: insights from the CHAMP‐HF registry. ESC Heart Failure 2021; 8: 710–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Rate of patient initiation on ARNI.
Figure S2. Rate of patient discontinuation of ARNI.
Figure S3. KCCQ Overall Summary Score Health Status Trajectory in Patients Initiated on ARNI vs Not Through 18‐months Following Initiation.
Figure S4. KCCQ Domain Health Status Trajectories in Patients Initiated on ARNI vs Not Through 18‐months Following Initiation.
Appendix S1. 5‐point Responder Analysis.


