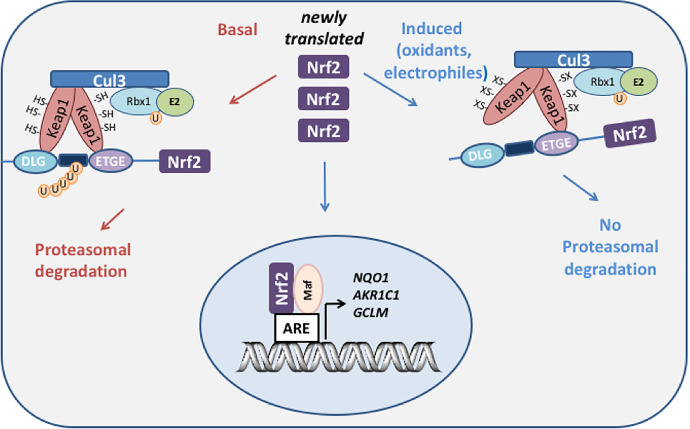
Fig. 2.
NRF2-Keap1 regulation of human AKR genes. Under basal conditions, the transcription factor Nrf2 is sequestered by Keap1 which acts as an adaptor program for culin 3 (cul 3) ubiquitin ligase which leads to the ubiquitination of Nrf2 and its proteasomal degradation. Upon exposure to oxidative or electrophilic stress, cysteine residues on Keap1 become either oxidized or covalently modified which disrupts the interaction with Nrf2 and prevents its ubiquitination. Under these conditions, newly synthesized Nrf2 evades proteasomal degradation, translocates to the nucleus where it binds to its heterodimeric partner small maf; and the resulting dimer binds to ARE in the promoters of genes that detoxify the stress signal, e.g., AKR1C, NQO1, and γ-glutamylcysteine synthetase (GCLM).