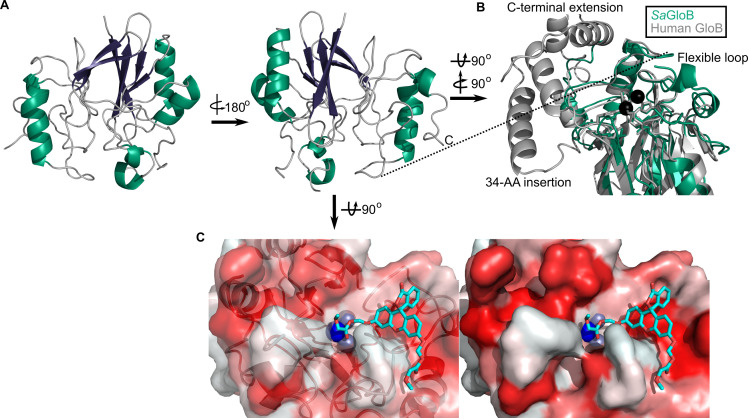
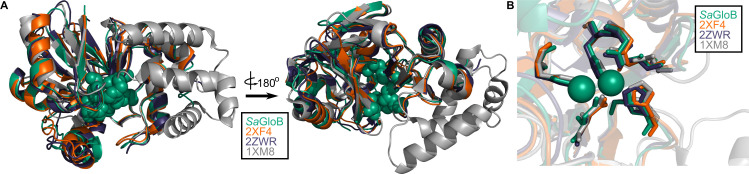
Figure 5. Three-dimensional structure of GloB.
(A) Overall fold, a-alpha helices colored in green and β-strands colored in purple. (B) Comparison of SaGloB (green) and human GloB (gray). (C) Docking of the substrate 1O (sticks) in the active site of GloB. Left, partial cartoon view; right, surface view. White represents hydrophilic residues, whereas red represents hydrophobic residues. Zn ions indicated as silver spheres; water indicated as blue sphere.