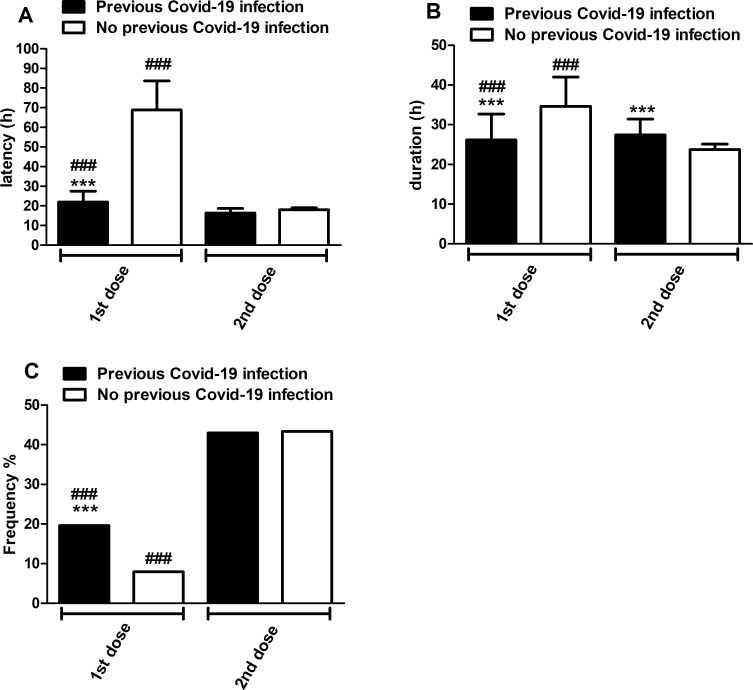
Figure 4.
Onset (panel A) and duration (panel B) of adverse effects and frequency of symptomatic drug intake (panel C) after the first and second doses of Pfizer/BioNTech COVID-19 vaccine stratified by previous and non-previous COVID-19 infection. Latency and duration are expressed as mean±SD in hours, while the frequency of symptomatic drug intake after vaccination is expressed as a percentage of previous and non-previous COVID-19 infected subjects (respectively 291 and 1739; panel C). Statistical analyses of latency and duration were performed with the Student t-test, while a comparison of frequencies was performed with the χ2 test. ***p<0.001 versus non-previous COVID-19 infection; ###p<0.001 versus second dose.