Abstract
Background
Biopsies are widely used for diagnosing metastatic tumors in the bone and soft tissues; however, their usefulness and limitations remain unclear.
Patients and methods
Biopsies of patients (13 men, 8 women, mean age 76 years) with metastatic tumors in the bone (19 patients) and soft tissues (2 patients) were reviewed retrospectively. Investigators surveyed the lesion sites, medical histories, Eastern Cooperative Oncology Group (ECOG) Performance Status (PS), biopsy sites, methods, comorbidities, diagnoses, treatments, and outcomes.
Results
Five patients had multiple lesions, and 16 patients had one lesion. The ECOG PS scores were PS0 (11 patients), PS1 (7 patients), PS2 (2 patients), and PS3 (1 patient). Biopsy sites included pelvic bone (6 cases), rib bone (5 cases), spinal vertebra (7 cases), soft tissue of the shoulder (2 cases), and inner retroperitoneum (1 case). Diagnostic methods included open biopsy (8 patients), core needle biopsy under general (7 patients) or local (3 patients) anesthesia, and computed tomography–guided core needle biopsy under local anesthesia (3 patients). Histology indicated hematological malignancies (9 cases); breast cancer (3 patients); lung cancer, renal cell cancer, cancer of unknown primary (2 cases each); prostate cancer, endometrial (uterine) cancer, and myxoid liposarcoma (1 case each). The primary site identification rate was 90.5%. Outcomes included three patients “dead of disease.“
Conclusion
Biopsies are useful for early diagnosis and for the scrutiny of primary lesions of metastatic bone and soft tissue tumors. If the primary tumor is still unknown after biopsy, evidence-based treatment should be initiated promptly.
Keywords: Biopsy, Metastatic tumors, Bone and soft tissue tumors
Highlights
-
•
1. Biopsy is essential and useful for the close examination of metastatic bone and soft tissue tumors.
-
•
It is better to use minimally invasive biopsy methods and biopsy sites.
-
•
For cancers of unknown primary, the primary site can be identified by tracing the patient's history.
1. Introduction
Nearly one in three patients with advanced malignancy have distant metastases at the time of clinical diagnosis [1]. Bone is the third most frequent site of metastasis for a wide range of solid cancers, including lung, breast, prostate, colorectal, thyroid, gynecologic cancers, and melanoma. Approximately 70% of patients with metastatic prostate and breast cancers have bone metastases [2]. Additionally, soft tissue metastases are rare, but may present as an initial finding [3,4]. These facts indicate that the metastasis of cancer to musculoskeletal sites has clinical significance.
Biopsies of the bone and soft tissues are often performed to confirm the primary site of cancer [4,5]. There are various types of biopsy techniques, including needle biopsy, incisional biopsy, and excisional biopsy [[5], [6], [7]]. Although some evidence exists on the effectiveness of biopsies for diagnosis, biopsies do not lead to definitive diagnoses [8]. Thus, the utility and limitations of biopsy procedures for the diagnosis of metastatic bone and soft tissue tumors have remained unclear [9]. In the current study, we conducted a retrospective analysis using data of patients treated at our department for bone or soft tissue metastases, wherein we clarified the clinical biopsy results in detail in an effort to determine the usefulness and limitations of using biopsy procedures as a diagnostic tool.
2. Patients and Methods
We retrospectively reviewed the cases of 21 patients at our hospital who had undergone biopsy procedures for metastatic tumors in the bone and soft tissues to confirm the diagnosis of the primary lesions. Data from 13 men and 8 women were included in the analysis. Biopsies had been performed on 19 metastatic bone lesions and 2 metastatic soft tissue lesions. The mean follow-up period was 6 months (range, 1–63 months). We surveyed lesion sites, lesion types, medical histories, Eastern Cooperative Oncology Group (ECOG) performance status (PS) [10], biopsy sites, biopsy methods, diagnoses, complications after biopsy, treatment modalities, and outcomes. Medical history was obtained by interviewing each patient during the outpatient visit. Imaging examinations were conducted at the main treatment department; additional imaging examinations, especially computed tomography (CT) imaging for biopsy, were conducted as necessary when the patient visited our department. All biopsies had been performed for confirming the diagnosis of the primary lesions.
3. Results
Patient characteristics are presented in Table 1. The mean age was 76 years (range: 43–92 years). Clinical images depicted 5 patients with multiple metastases, and 16 patients with single metastatic lesions. The biopsy sites were as follows: pelvic bone (5 cases), rib bone (5 cases), lumbar vertebra (5 cases), thoracic vertebra and soft tissue of the shoulder (2 cases each), and pubic bone and inner retroperitoneum (1 case each).
Table 1.
Clinical characteristics of patients in the current study.
| Patient no. | Sex | Age | Lesion site | Lesion type | History | PS | Biopsy site | Biopsy method | Diagnosis | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 68 | Both ribs | Osteolytic | Tuberculosis | 0 | Right rib | Open biopsy | Adenocarcinoma | CTp, RT | DOD |
| 2 | M | 79 | Right rib | Mixed | – | 0 | Right rib | Open biopsy | Plasmacytoma | CTp | AWD |
| 3 | M | 76 | Right 9th rib and left 9th rib | Osteolytic | 0 | left 9th rib | Open biopsy | Multiple myeloma | CTp | AWD | |
| 4 | F | 78 | 3rd lumbar vertebra | Osteolytic | HT, Breast Cancer | 1 | 3rd lumbar vertebra | Needle (vertebra) | Breast cancer | CTp | AWD |
| 5 | M | 92 | Left soft tissue shoulder | – | HT, DM, HT, Benign prostatic hyperplasia | 1 | Left soft tissue shoulder | Needle | Lung cancer | RT | AWD |
| 6 | F | 82 | 9th thoracic vertebra | Mixed | MR | 2 | 9th thoracic vertebra | Needle (vertebra) | DLBCL | RT | AWD |
| 7 | M | 76 | 12th thoracic vertebra | Mixed | – | 2 | 12th thoracic vertebra | Needle (vertebra) | DLBCL | RT | AWD |
| 8 | M | 60 | 5th lumbar vertebra | Osteolytic | – | 0 | 5th lumbar vertebra | Needle (vertebra) | LCH | CTp | CDF |
| 9 | F | 69 | Right 4th rib | Osteolytic | – | 0 | Right 4th rib | Open biopsy | Multiple myeloma | – | CDF |
| 10 | M | 74 | Right pubis, lung, lumbar vertebra | Osteolytic | HT, DM | 0 | Right pubis | Open biopsy | Renal cancer | Immunotherapy | AWD |
| 11 | M | 80 | Pelvic | Osteolytic | HT, HL | 1 | Pelvic | CT-guided needle biopsy | Prostate cancer | RT | AWD |
| 12 | F | 82 | pelvic | Osteolytic | HT, DM | 1 | Pelvic | Open biopsy | Endometrial cancer | CTp | AWD |
| 13 | M | 75 | Left 9th rib | Osteolytic | DM | 0 | Left 9th rib | Open biopsy | Multiple myeloma | CTp, RT | AWD |
| 14 | F | 72 | Inner retroperitoneum | Osteolytic | HT, Breast Cancer | 1 | Inner retroperitoneum | Open biopsy | Breast Cancer | CTp | AWD |
| 15 | F | 43 | 1st lumbar vertebra | Osteolytic | Breast Cancer Colon Cancer | 0 | 1st lumbar vertebra | Needle (vertebra) | Breast Cancer | CTp | AWD |
| 16 | F | 77 | Left soft tissue of shoulder | – | DM | 0 | Left soft tissue of shoulder | Needle | DLBCL | CTp, RT | AWD |
| 17 | M | 77 | Left pubis | Osteoblastic | HT | 1 | Left pubis | CT-guided needle biopsy | Renal cancer | Immunotherapy | AWD |
| 18 | M | 72 | Pelvic | Osteolytic | Appendicitis | 1 | Pelvic | CT-guided needle biopsy | Liposarcoma | Heavy ion beam therapy | AWD |
| 19 | M | 72 | 3rd lumbar vertebra | Osteolytic | DM | 0 | 3rd lumbar vertebra | Needle (vertebra) | Lung cancer | – | DOD |
| 20 | M | 83 | Multiple pelvic | Osteolytic | Hepatitis type C | 0 | Pelvic | Open biopsy | Lymphoma | RT | AWD |
| 21 | F | 74 | 7th cervical vertebra, 1st and 2nd thoracic vertebra, pelvic | Osteolytic | HT, Uterus Cancer, pyelonephritis | 3 | Pelvic | Needle | CUP | RT | DOD |
AWD, alive with disease; CUP, cancer of unknown primary; CT, computed tomography; CTp, chemotherapy; DLBCL, diffuse large B-cell lymphoma; DM, diabetes mellitus; DOD, dead of disease; F, female; HL, hyperlipidemia; HT, hypertension; LCH, Langerhans cell histiocytosis; M, male; MR, mitral valve regurgitation; PS, performance status; RT, radiation therapy.
Lytic lesions were observed in 15 cases, an osteoblastic lesion was observed in 1 case, mixed lesions were observed in 3 cases, and soft tissue lesions were observed in 2 cases.
Histological findings included 3 cases of diffuse large B-cell lymphoma, 3 cases of multiple myeloma, 3 cases of breast cancer, 2 cases of lung cancer, 2 cases of renal cell cancer, 2 cases of cancer of unknown primary, 1 case of plasmacytoma, 1 case of Langerhans cell histiocytosis, 1 case of prostate cancer, 1 case of endometrial cancer of the uterus, and 1 case of myxoid liposarcoma. The ECOG PS scores were as follows: PS0, 11 patients; PS1, 7 patients; PS2, 2 patients; and PS3, 1 patient. The following biopsy techniques had been used: core needle biopsy under general anesthesia (6 cases), open biopsy (9 cases), core needle biopsy under local anesthesia (3 cases), and CT-guided core needle biopsy under local anesthesia (3 cases). No complications were observed following the biopsy. The primary site identification rate was 90.5%. The treatment instituted was chemotherapy for 7 cases, radiation therapy for 6 cases, chemotherapy and radiation therapy for 3 cases, immunotherapy for 2 cases, and heavy particle radiation for 1 case. The outcome was 2 cases of continuous disease free (CDF), 16 cases of alive with disease (AWD), and 3 cases of dead of disease (DOD).
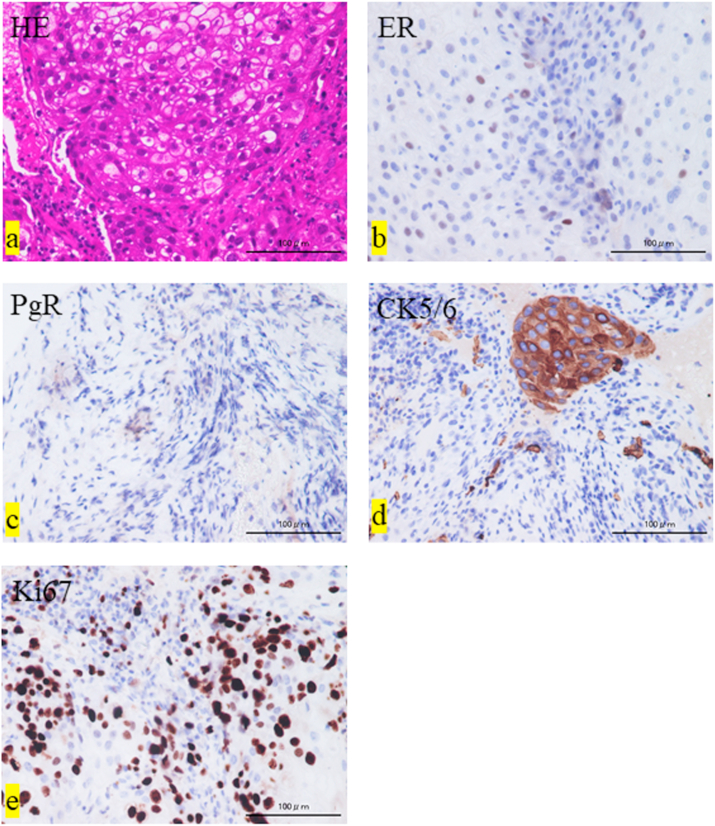
In two cases, the primary tumor could not be identified by biopsy; both were adenocarcinomas. In one of those cases, the patient's medical history and immunostaining of the primary lesion confirmed that the primary tumor was breast cancer (Patient 1, Table 1). The biopsy specimen showed sheet-like growth of atypical cells with a broad cytoplasm that was pale to acidophilic staining (Fig. 1a). Immunostaining analysis revealed estrogen- and progesterone-positive cells (Fig. 1b and c), cytokeratin 5/6–positive cells (Fig. 1d), and a high Ki-67 positivity rate (Fig. 1e). Three cases of DOD were observed as outcomes.
Fig. 1.
Histological findings. (a) Hematoxylin and eosin (HE) staining. (b) Immunostaining shows estrogen receptor (ER)-positive cells, (c) progesterone receptor (PgR)-positive cells, (d) cytokeratin (CK)5/6–positive cells, and (e) Ki-67-positive cells (>30% positivity rate).
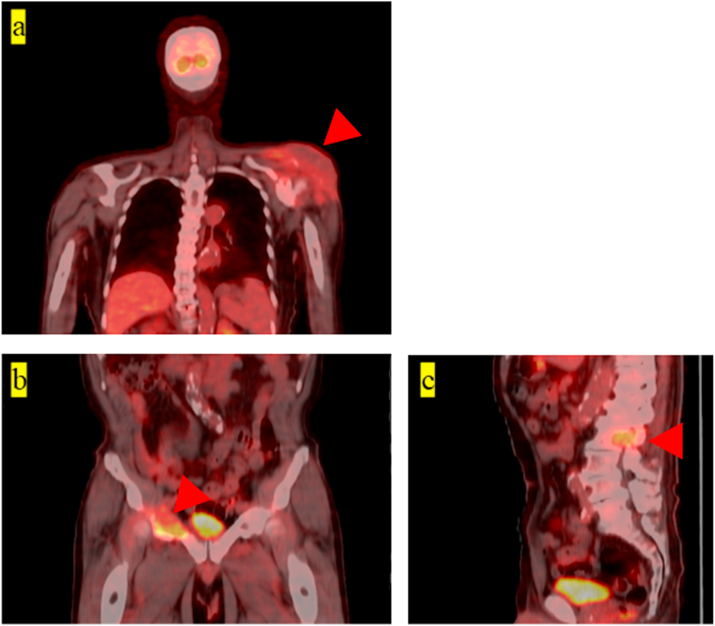
One representative case of a single lesion and one of multiple lesions are described as follows. A 77-year-old woman underwent core needle biopsy of a soft tissue mass on her left shoulder (Fig. 2a: single lesion case; Patient 16, Table 1). Pathological results revealed diffuse large B-cell lymphoma.
Fig. 2.
Representative cases of a single lesion and multiple lesions. Patient 16, single lesion: accumulation can be observed on the left shoulder (a). Patient 10, multiple lesions: accumulation can be observed on the right pubis (b) and lumbar vertebra (c). Red arrows indicate the lesions. . (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
A 74-year-old man had lytic lesions on the right pubis and lumbar vertebra; an incisional biopsy was performed at the right pubis (Fig. 2b and c: multiple lesions case; Patient 10, Table 1). Pathological results revealed renal carcinoma.
4. Discussion
Metastases to the bone reportedly originate from the major sites of primary cancer (i.e., breast, prostate, lung, and thyroid) and most commonly affect the spine (36.0%), hip (32.8%), and long bones (18.3%) [11,12]. Core needle biopsy and fine needle aspiration are useful methods for diagnosing metastatic tumors, since they are safe, accurate, minimally invasive, and have high diagnostic significance [5]. Among bone metastases, the spine is the most frequently aspirated site (49%), followed by the ilium, sacrum, mandible, ribs, and femur [13]. Unfortunately, sample volumes obtained from needle biopsies are sometimes insufficient [11]. Another diagnostic method, CT-guided percutaneous core needle biopsy, is considered a safe and effective technique: only 3 complications (1.6%) have been previously reported, including fracture, paralysis with functional impairment, and needle breakage requiring surgical removal [11]. In contrast to the abovementioned techniques, incisional biopsies have been shown to increase the risk of lesion metastases [14].
In our study, ribs were a relatively common sample collection site (5/21 cases). In all cases, the sample volume was sufficient and useful for diagnosis. The PS scores before biopsy did not affect the choice of biopsy method nor were they associated with complications. Despite the lack of complications, we believe that less invasive biopsy methods should be considered.
Previous studies have shown that the primary site identification rate for metastatic bone tumors is 94.1–98.4% [2,11,15], with breast (32.1%) and prostate (11.8%) being the most common primary sites [11]. In our study, the primary site identification rate for such tumors was 90.5% (19/21 cases). This is generally considered effective for the detection of primary lesions. Histological results indicated that the most common primary lesion was hematologic malignancy (9/21 cases).
For soft tissue tumors, primary site identification faces certain issues. Histologically, the three most common types of epithelial malignancies are adenocarcinoma, undifferentiated carcinoma, and poorly differentiated carcinoma. The remaining 10% are squamous cell carcinoma, neuroendocrine carcinoma, and rare histological types [16]. Definitive diagnosis of the primary tumor depends largely on the immunostaining technique used [17]. Although immunostaining with cytokeratin 7 and 20 can narrow down the list to some extent, it is often difficult to estimate the primary organ of adenocarcinoma from histology alone [17]. Similarly, in the current study, the primary site for two adenocarcinomas could not be confirmed via biopsy; in one of those cases, the primary site was confirmed by reviewing the patient's history. For such cases, the diagnosis should be based on clinical features and findings, including past medical history.
Cancer of unknown primary (CUP) accounts for 2–3% of all epithelial malignancies [18]. Typical treatment for CUP includes a combination of platinum and paclitaxel; however, the level of evidence for this treatment strategy is low [18]. Gemcitabine (alone or in combination with platinum/paclitaxel) has recently been used as alternative therapy for the treatment of CUP [18]; nevertheless, the prognosis for CUP remains extremely poor [19]. In the current study, one patient was treated with chemotherapy consisting of carboplatin and paclitaxel, but the outcome at the final follow-up period (49 months after biopsy) was DOD. The other DOD patient in the study had poor PS and had undergone palliative irradiation only, resulting in death from disease at 3 months following the biopsy. Therefore, CUP treatment should be initiated as early as possible.
4.1. Limitations
The current study's limitations include its small number of cases and the possibility of bias owing to its retrospective descriptive design. The follow-up period was short; however, considering the poor prognosis of metastatic bone tumors and that our median follow-up period was six months, we believe that the length of the study period was adequate for determining its objectives.
5. Conclusion
While some of our patients’ primary lesions were identified based on clinical rather than biopsy results, and although a few of the primary lesions remained unidentified, our findings do support that biopsies are generally useful for diagnosing bone and soft tissue metastases and for identifying their primary site. Therefore, we believe that tumor biopsies (together with close interdepartmental collaboration) can improve patient outcomes by enabling prompt diagnosis and appropriate treatment. Future analytical studies are recommended to explore the full potential of this diagnostic tool.
Ethical approval
This case report was conducted according of the Declaration of Helsinki of 1975 (revised in 2013), and approved by the Ethics Committee of Kindai University Hospital (approval number: 31–253; Osaka, Japan; and date of approval February 8, 2020).
Sources of funding
None.
Author contribution
Conceptualization: Kazuhiko Hashimoto, Shunji Nishimura, Tomohiko Ito, Masao Akagi. Methodology: Kazuhiko Hashimoto, Shunji Nishimura, Naohiro Oka, Masao Akagi. Software: Kazuhiko Hashimoto, Tomohiko Ito, Naohiro Oka, Shunji Nishimura. Validation: Shunji Nishimura, Tomohiko Ito, Naohiro Oka, Masao Akagi. Formal analysis: Shunji Nishimura, Naohiro Oka, Masao Akagi. Investigation: Kazuhiko Hashimoto, Tomohiko Ito, Naohiro Oka, Shunji Nishimura. Data curation: Kazuhiko Hashimoto, Shunji Nishimura, Tomohiko Ito, Masao Akagi. Writing – original draft: Kazuhiko Hashimoto, Shunji Nishimura, Tomohiko Ito, Naohiro Oka, Masao Akagi. Writing – review & editing: Kazuhiko Hashimoto, Shunji Nishimura, Tomohiko Ito, Naohiro Oka, Masao Akagi. All authors have read and agreed to the published version of the manuscript.
Registration of research studies
Name of the registry:
The research to confirm prognosis of malignancies the Ethics Committee of Kindai University Hospital.
Unique Identifying number or registration ID: approval number: 31-253.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.med.kindai.ac.jp/rinsyo/
Guarantor
The Guarantor is Kazuhiko Hashimoto.
Consent
Informed consent was obtained from all subjects involved in the study.
Declaration of competing interest
All authors declare no conflicts of interest.
References
- 1.Tomuleasa C., Zaharie F., Muresan M.S. How to diagnose and treat a cancer of unknown primary site. J. Gastrointestin. Liver. Dis. 2017;26:69–79. doi: 10.15403/jgld.2014.1121.261.haz. https://doi:10.15403/jgld.2014.1121.261.haz [DOI] [PubMed] [Google Scholar]
- 2.Fornetti J., Welm A.L., Stewart S.A. Understanding the bone in cancer metastasis. J. Bone Miner. Res. 2018;33:2099–2113. doi: 10.1002/jbmr.3618. https://doi:10.1002/jbmr.3618 [DOI] [PubMed] [Google Scholar]
- 3.Plaza J.A., Perez-Montiel D., Mayerson J. Metastases to soft tissue: a review of 118 cases over a 30-year period. Cancer. 2008;112:193–203. doi: 10.1002/cncr.23151. https://doi:10.1002/cncr.23151 [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto K., Nishimura S., Akagi M. Lung adenocarcinoma presenting as a soft tissue metastasis to the shoulder: a case report. Medicina. 2021;57:181. doi: 10.3390/medicina57020181. https://doi:10.3390/medicina57020181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitsuyoshi G., Naito N., Kawai A. Accurate diagnosis of musculoskeletal lesions by core needle biopsy. J. Surg. Oncol. 2006;94:21–27. doi: 10.1002/jso.20504. https://doi:10.1002/jso.20504 [DOI] [PubMed] [Google Scholar]
- 6.Skrzynski M.C., Biermann J.S., Montag A. Diagnostic accuracy and charge-savings of outpatient core needle biopsy compared with open biopsy of musculoskeletal tumors. J. Bone. Joint. Surg. Am. 1996;78:644–649. doi: 10.2106/00004623-199605000-00002. https://doi:10.2106/00004623-199605000-00002 [DOI] [PubMed] [Google Scholar]
- 7.Dupuy D.E., Rosenberg A.E., Punyaratabandhu T. Accuracy of CT-guided needle biopsy of musculoskeletal neoplasms. AJR Am. J. Roentgenol. 1998;11:759–762. doi: 10.2214/ajr.171.3.ajronline_171_3_001. https://doi:10.2214/ajr.171.3.ajronline_171_3_001 [DOI] [PubMed] [Google Scholar]
- 8.Pavlidis N., Briasoulis E., Hainsworth J. Diagnostic and therapeutic management of cancer of an unknown primary. Eur. J. Canc. 2003;39:1990–2005. doi: 10.1016/s0959-8049(03)00547-1. https://doi:10.1016/s0959-8049(03)00547-1 [DOI] [PubMed] [Google Scholar]
- 9.Bauer H.C. Controversies in the surgical management of skeletal metastases. J. Bone. Joint. Surg. Br. 2005;87:608–617. doi: 10.1302/0301-620X.87B5.16021. https://doi:10.1302/0301-620X.87B5.16021 [DOI] [PubMed] [Google Scholar]
- 10.Neeman E., Gresham G., Ovasapians N. Comparing physician and nurse eastern cooperative Oncology Group performance status (ECOG-PS) ratings as predictors of clinical outcomes in patients with cancer. Oncol. 2019;24:e1460–e1466. doi: 10.1634/theoncologist.2018-0882. https://doi:10.1634/theoncologist.2018-0882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maciel M.J., Tyng C.J., Barbosa P.N. Computed tomography-guided percutaneous biopsy of bone lesions: rate of diagnostic success and complications. Radiol. Bras. 2014;47:269–274. doi: 10.1590/0100-3984.2013.0004. https://doi:10.1590/0100-3984.2013.0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piccioli A., Maccauro G., Spinelli M.S. Bone metastases of unknown origin: epidemiology and principles of management. J. Orthop. Traumatol. 2015;16:81–86. doi: 10.1007/s10195-015-0344-0. https://doi:10.1007/s10195-015-0344-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bommer K.K., Ramzy I., Mody D. Fine-needle aspiration biopsy in the diagnosis and management of bone lesions: a study of 450 cases. Cancer. 1997;81:148–156. doi: 10.1002/(sici)1097-0142(19970625)81:3<148::aid-cncr4>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 14.Alieva M., van Rheenen J., Broekman M.L.D. Potential impact of invasive surgical procedures on primary tumor growth and metastasis. Clin. Exp. Metastasis. 2018;35:319–331. doi: 10.1007/s10585-018-9896-8. https://doi:10.1007/s10585-018-9896-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hage W.D., Aboulafia A.J., Aboulafia D.M. Incidence, location, and diagnostic evaluation of metastatic bone disease. Orthop. Clin. N. Am. 2000;31:515–528. doi: 10.1016/s0030-5898(05)70171-1. https://doi:10.1016/s0030-5898(05)70171-1 [DOI] [PubMed] [Google Scholar]
- 16.Yakushiji S., Ando M., Yonemori K. Cancer of unknown primary site: review of consecutive cases at the National Cancer Center Hospital of Japan. Int. J. Clin. Oncol. 2006;11:421–425. doi: 10.1007/s10147-006-0599-9. https://doi:10.1007/s10147-006-0599-9 [DOI] [PubMed] [Google Scholar]
- 17.Chu P., Wu E., Weiss L.M. Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: a survey of 435 cases. Mod. Pathol. 2000;13:962–972. doi: 10.1038/modpathol.3880175. https://doi:10.1038/modpathol.3880175 [DOI] [PubMed] [Google Scholar]
- 18.Bochtler T., Löffler H., Krämer A. Diagnosis and management of metastatic neoplasms with unknown primary. Semin. Diagn. Pathol. 2018;35:199–206. doi: 10.1053/j.semdp.2017.11.013. https://doi:10.1053/j.semdp.2017.11.013 [DOI] [PubMed] [Google Scholar]
- 19.Moran S., Martínez-Cardús A., Sayols S. Epigenetic profiling to classify cancer of unknown primary: a multicentre, retrospective analysis. Lancet Oncol. 2016;17:1386–1395. doi: 10.1016/S1470-2045(16)30297-2. https://doi:10.1016/S1470-2045(16)30297-2 [DOI] [PubMed] [Google Scholar]