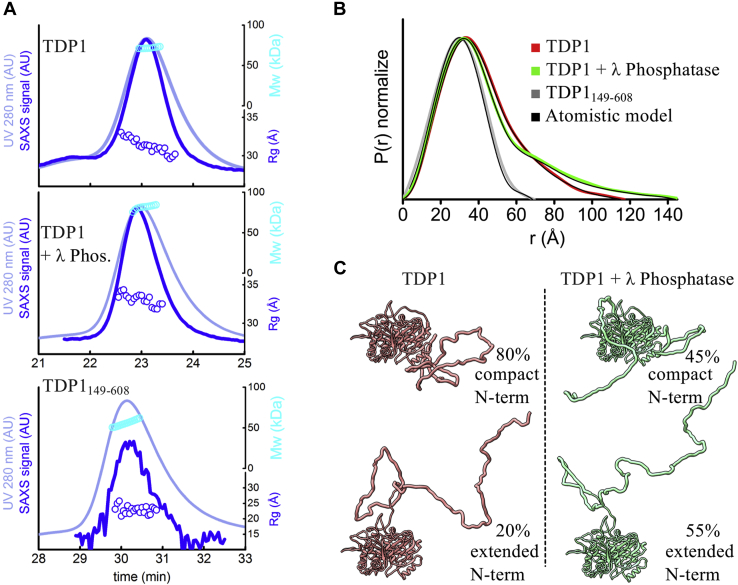
Figure 4.
TDP1 N-terminus adopts an open conformation upon protein dephosphorylation.A, SEC- SAXS-MALS chromatographs for TDP1, TDP1 with λ phosphatase, and TDP1149–608. Solid lines represent the UV 280 nm (light blue) or SAXS signal (blue) in arbitrary units, while symbols represent molecular mass determined by MALS (cyan) and Rg values for the principal SEC peak (blue) versus elution time. SEC-SAXS-MALS results show that TDP1 is a monomer. B, normalized pair distribution [P(r)] functions of TDP1 and TDP1 with λ phosphatase matching theoretical P(r) functions of atomistic models (black) shown in panel C. Normalized P(r) functions of TDP1149–608 matching theoretical P(r) functions of TDP1 crystal structure (PDB 1JY1). C, ensemble models of two conformers of TDP1 and λ phosphatase-treated TDP1 that were used to fit experimental SAXS curves shown in Figure S8 and P(r) functions shown in panel B.