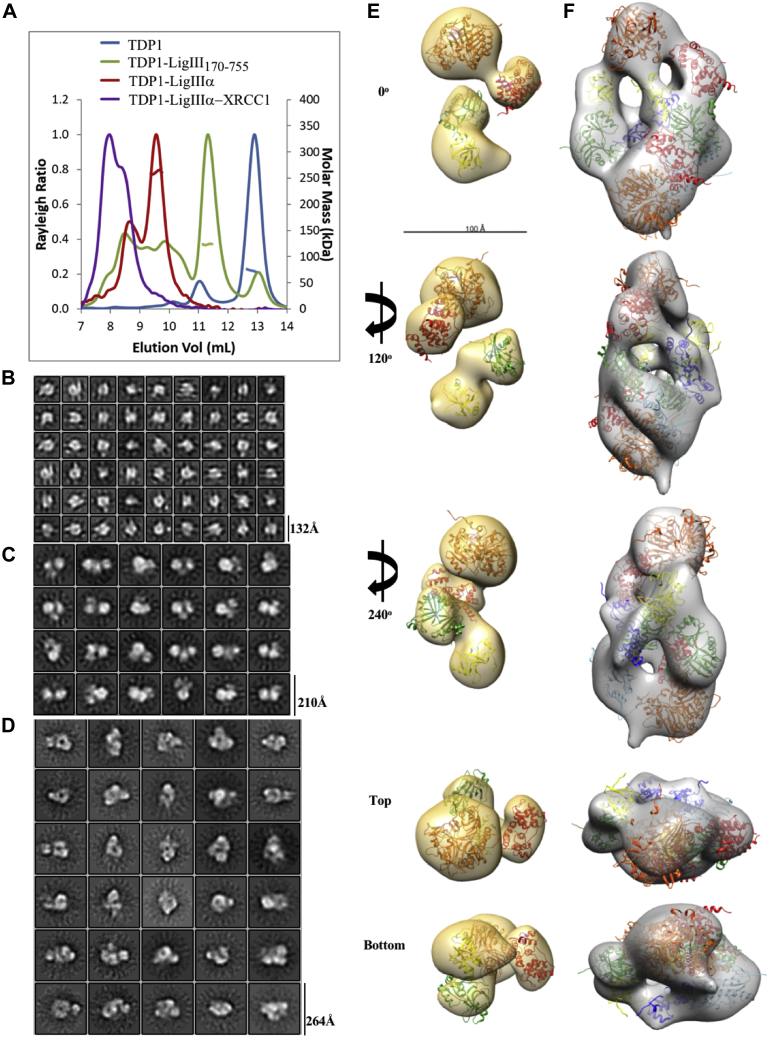
Figure 5.
SEC-MALS and EM of TDP1-LigIII170–755and TDP-LigIIIα.A, SEC-MALS analysis of TDP1 alone (blue) and in complex with LigIII170–755 (green), LigIIIα (red), or LigIIIα-XRCC1 (purple). The resultant molecular mass is plotted in the same color, where applicable. Molecular mass was not obtained for the tripartite complex due to its void volume elution. Negative-stain EM 2D classification of TDP1 alone (B), TDP1-LigIII170–755 (C), and TDP1-LigIIIα (D) shows homogeneous particles clustered in several orientations. The box size is indicated for each data set classification. The TDP1-LigIII170–755 (E) and TDP1-LigIIIα (F) 3D maps are shown rotated relative to 0°. Based on stoichiometry derived from molecular mass determination, crystal structures of TDP1149–608 (PDB 1JY1, orange) and LigIII170–755 (PDB 3L2P) were docked in a 1:1 ratio (E) or a 2:2 ratio (F). The DBD (red), NTase (green), and OB-fold (yellow) domains of LigIII170–755 were separated to fit into the EM map and the DBD was placed adjacent to TDP1149–608. For full-length LigIIIα, two ZnF domains (PDB 1UW0, light blue) and its BRCT homodimer (PDB 3PC8, navy blue) were also docked. The BRCT homodimer was placed in the center of the volume as it most likely mediates dimerization between two molecules of LigIIIα.