Abstract
Antibiotic overuse in poultry husbandry poses a potential threat to meat safety and human health. Lauric acid (LA) is a primary medium-chain fatty acid (MCFA) with a strong antibacterial capacity. The goal of this study was to evaluate the beneficial effects of LA on the growth performance, immune responses, serum metabolism, and cecal microbiota of broiler chickens. One-day-old male Ross 308 broilers were randomly divided into 4 groups: CON, fed a basal diet; ANT, a basal diet supplemented with 75 mg/kg antibiotic; LA500, a basal diet supplemented with 500 mg/kg LA; LA1000, a basal diet supplemented with 1000 mg/kg LA. The feeding period was 42 d. The results showed that LA significantly improved broiler growth and immune functions, as evidenced by increased body weight (BW) and average daily gain (ADG), enhanced intestinal mucosal barrier, upregulated immunoglobulins (IgA, IgM, and IgY), and downregulated inflammatory cytokines (IL-1β, IL-6, TNF-α, IL-4, and IL-10) (P < 0.05). HPLC/MS-based metabolome analysis revealed that the serum metabolites in the LA group differed from those of CON and ANT groups. LA markedly decreased the abundance of phosphatidylcholines (PCs), increased lysophosphatidylcholines (LysoPCs), and inhibited the sphingolipid metabolism pathway, indicating its capacity to modulate lipid metabolism. 16S rRNA sequencing indicated that LA significantly altered cecal microbiota composition by reducing Phascolarctobacterium, Christensenellaceae_R-7_group, and Bacteroides, and increasing Faecalibacterium and Ruminococcaceae_UCG-014 (P < 0.05). Furthermore, Spearman correlation analysis revealed that changes in metabolism and microbiota were highly correlated with the growth and immune indices; strong links were also found between lipid metabolism and microbial composition. Taken together, LA promotes broiler growth and immune functions by regulating lipid metabolism and gut microbiota. The above findings highlight the substantial potential of LA as a supplement in poultry diets and provide a new strategy to reduce antibiotic usage and improve food safety.
Key words: lauric acid, broiler, growth and immunity, lipid metabolism, gut microbiota
INTRODUCTION
In-feed antibiotics (IFAs) have been incorporated into poultry diets for decades because of their favorable effects on growth performance and disease prevention (Arias and Koutsos, 2006; Dahiya et al., 2006). However, the continual feeding of IFAs can lead to the development and propagation of antibiotic resistance to many pathogenic bacteria and poses a potential threat to food safety, the environment, and public health (Cheng et al., 2014). Consequently, IFAs have been severely limited or completely abolished in many countries. Lauric acid (LA) is a primary medium-chain fatty acid (MCFA) with 12 carbon atoms that exists in many natural products, particular in the coconut oil (Dayrit, 2015). In addition to its modulation of metabolic and immune functions (Dayrit, 2015; Lappano et al., 2017), LA possesses significant antimicrobial properties, which has led to its gradual use as a feed additive (Hanczakowska, 2017; Omonijo et al., 2018). Studies have shown that LA significantly increases feed intake and improves gut development and nutrient absorption, enhancing animal growth (Simo-Mirabet et al., 2017). LA could protect chickens against Clostridium perfringens and Eimeria-induced necrotic enteritis (Yang et al., 2019). Although studies have investigated the beneficial effects of LA in poultry, the underlying mechanisms remain unclear.
Gut microbiota is a diverse ecosystem inhabiting the gastrointestinal tract and comprises at least 1013–1014 microbial cells and over 2,000 distinct species (Flint et al., 2012). Factors such as shifts in host diets, drugs, and stimuli may alter the microbial composition, thereby altering the physiological activities of the host (Maier et al., 2018). The balance of this microbiome is crucial to enhance nutrient metabolism, the intestinal mucosal barrier, and immune functions. It has been reported that beneficial intestinal microbes can secrete enzymes that are not encoded by the host genome to degrade polysaccharides, synthesize vitamins, and produce short-chain fatty acids (SCFAs) and tryptophan metabolites (Agus et al., 2018; Rowland et al., 2018). These metabolites transfer to various organs and tissues through blood circulation, providing energy and regulating various physiological activities.
LA has recently been reported to modulate the composition and distribution of the gut microbiota. Studies have shown that LA has low antimicrobial activity against commensal lactic acid bacteria but strong activity against pathogenic Bacteroides and Clostridium (Matsue et al., 2019). In a piglet model, LA administration significantly decreased the abundance of Prevotella and increased Selenomonadales (Sol et al., 2019). Glycerol monolaurate significantly increased Fusobacterium, Mucispirillum, and norank_f_Pedosphaeracea in aged hens (Liu et al., 2020). Although the ability of LA to alter microbiota has been shown, its contribution to host metabolism and health remains unclear. This study first evaluated the potential of LA in improving the growth performance and immune functions of broiler chickens. Then, HPLC/MS-based metabolomics were then used to detect the changes in serum metabolites and 16S rRNA sequencing to analyze the gut microbial composition. Spearman correlation analysis was conducted to confirm the key role of the altered metabolism and microbiota in LA-mediated beneficial effects.
MATERIALS AND METHODS
Animal Experimental Design
The experimental procedures were approved by the Animal Care and Use Committee of Zhejiang A&F University and performed in accordance with the Animals in Research: Reporting In Vivo Experiments (ARRIVE) guidelines for reporting animal research (Kilkenny et al., 2012). Ethical approval for animal survival was provided by the Animal Ethics Committee of Zhejiang A&F University. A total of 384 one-day-old male Ross 308 broiler chickens were randomly divided into 4 groups with 8 replicates. Each repetition was allocated to a cage (12 birds per cage) that had raised wire floors. The treatment groups were as follows: broilers fed a basal diet (CON), a basal diet supplemented with 75 mg/kg aureomycin (ANT), a basal diet supplemented with 500 mg/kg LA (LA500), and a basal diet supplemented with 1000 mg/kg LA (LA1000). LA was provided by Vegamax Biotechnology Co., Ltd. (Huzhou, China). The feeding period was 42 d. Broilers were housed in an air-conditioned room, and the temperature was maintained at 20.0 to 25.0°C. Feed and water were provided ad libitum. Broiler management was performed according to the guidelines of Aviagen (2016). The basal diet was formulated to meet the nutrient requirements suggested by the National Research Council (1998), and the recipes and nutrients are listed in Table S1.
Evaluation of Growth Performance
To evaluate the growth performance of broilers, body weight (BW) of 8 repetitions (12 birds per repetition) in each group was measured on d 1, 21, and 42, and average daily gain (ADG) was calculated. The feed intake of each period was measured to calculate the average daily feed intake (ADFI) and feed conversion ratio (FCR).
Sample Collection
At the end of the feeding trial, one broiler was randomly taken from 8 repetitions in each group (N = 8 per group) and sacrificed by an injection of sodium pentobarbital (20 mg/kg BW) (Shao et al., 2019). Blood was collected from the wing vein, and serum samples were obtained by centrifuging at 4000 g for 15 min. After dissection, intestinal tissues and cecal contents were collected. All samples were stored at -80°C until analysis.
Intestinal Morphological Analysis
Tissue samples from the jejunum and ileum were fixed in 10% buffered formalin saline, dehydrated, infiltrated with xylene, embedded in paraffin, sliced, and stained with hematoxylin and eosin (H&E). The tissue structure was observed using an optical microscope (Nikon, Tokyo, Japan). Villus height and crypt depth were measured at 10 visual fields of each intestinal sample in each group (N = 8 per group).
Analysis of Immune Factors in Serum
Levels of immunoglobulin A (IgA), immunoglobulin M (IgM), immunoglobulin Y (IgY), interleukin-1β (IL-1β), interleukin-6 (IL-6), tumor inflammatory factor-α (TNF-α), interleukin-4 (IL-4), and interleukin-10 (IL-10) were determined according to the manufacturer's instructions (Huamei Biological Engineering Research Institute, Wuhan, China), and measured with a multifunctional microplate reader (SynergyTM H1, BioTek, Winooski, VT, USA).
Untargeted HPLC/MS-based Metabolomics
Metabolites were performed on a Thermo UHPLC system equipped with an ACQUITY BEH C18 column (Waters, Milford, MA, USA) coupled to a Thermo UHPLC-Q Exactive Mass Spectrometer (Waters, Milford, USA). Briefly, metabolites were extracted from serum samples using a methanol:water (4:1, v/v) solution, homogenized, ultrasonicated, and allowed to stand for proteins to be precipitated. After centrifugation, the supernatant was transferred to sample vials for LC-MS/MS analysis. Quality control samples were prepared and tested in the same manner. Mass spectrometry detection was conducted in either the positive or negative ion mode (full scan mode from 70 to 1,050 m/z) with a capillary temperature of 400°C, spray voltage of 2.8 kV in negative mode and 3.5 kV in positive mode, sheath gas flow rate of 40 psi, and aus gas flow rate of 30 psi. Data acquisition was performed using the data-dependent acquisition mode.
Raw data were preprocessed by importing them into Progenesis QI 2.3 (Nonlinear Dynamics, Waters, Milford, MA, USA). At least 80% of the metabolic features were retained. After filtering and normalization, mass spectra of the metabolic features were identified using the accurate mass, MS/MS fragment spectra, and isotope ratio difference and searching the Human Metabolome Database (http://www.hmdb.ca/) and Metlin database (https://metlin.scripps.edu/). Multivariate statistical analysis was performed using the Majorbio Cloud Platform (https://cloud.majorbio.com) using ropls (Version1.6.2, http://bioconductor.org/packages/release/bioc/html/ropls.html) R package from Bioconductor. Principal component analysis (PCA) was performed using unit variance conversion. Partial least squares discriminant analysis (PLS-DA) was performed using Pareto scaling methods to differentiate groups. Two hundred permutation tests were conducted for the parameters R2 and Q2 to confirm the reliability of the model. Metabolite clustering analysis was conducted by averaging, hierarchical clustering, and Euclidean algorithms. Differential metabolites from the Top100 enriched compounds were analyzed using a one-way ANOVA and Tukey's test. The pathways were enriched based on KEGG (http://www. genome.jp/kegg/). Annotation analysis was conducted using scipy.stats (Python packages) (https://docs. scipy.org/doc/scipy/) using Fisher's exact test.
16S rRNA-based Microbiota Analysis
Microbial DNA from cecal contents was extracted using the E.Z.N.A. Soil DNA Kit (Omega Bio-tek, Norcross, GA according to the manufacturer's protocols. The bacterial 16S rRNA was amplified using the V3-V4 region primer 338F (5ʹ-ACTCCTACGGGAGG CACAG-3ʹ) and 806R (5ʹ-GGACTACHVGGGTWTCTAA T-3ʹ). After amplification and purification, the amplicons were pooled in equimolar amounts and paired-end sequenced using an Illumina MiSeq platform (Illumina, San Diego, CA, USA) according to the protocols of Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China).
Raw fastq files were quality-filtered using Trimmomatic and merged using FLASH 1.2.1 (https://ccb.jhu.edu/software/FLASH/index.shtml). Operational taxonomic units (OTUs) were clustered with a 97% threshold using UPARSE (version 7.1, http://drive5.com/uparse/). Statistical analysis was performed using the Majorbio Cloud Platform (https://cloud.majorbio.com). The taxonomy of each 16S rRNA gene sequence was analyzed using the RDP Classifier algorithm (http://rdp.cme.msu.edu/) with a confidence threshold of 70%. Mothur1.30.2 (https://www.mothur.org/wiki/ Download_mothur) was used to calculate the Shannon index. β-diversity, based on unweighted UniFrac distance, was calculated using QIIME1.9.1 (http://qiime.org/install/index.html), and statistical analysis was conducted using the ANOSIM method. Linear discriminant analysis (LDA) effect size (LEfSe) analysis was performed to identify biomarkers of microbial taxa based on an LDA score > 2.0 (http://huttenhower.sph.harvard.edu/galaxy/root?tool_id=lefse_upload).
VFA Analysis
The concentrations of VFAs were determined using headspace sampler gas chromatography (Agilent Technologies, Wilmington, DE, USA) according to previous studies (Thanh et al., 2009; Yang et al., 2019). The external standards of VFAs (acetic acid, propionic acid, butyric acid, isobutyric acid, valerate acid, and isovaleric acid) and metaphosphoric acid were purchased from Macklin Biochemical Co., Ltd. (Shanghai, China). One gram of cecal content was blended with 6% phosphorous acid (m/v, 1:4) and injected into Agilent Technologies GC7890 Network System, equipped with a 30 m × 0.25 mm × 0.25 μm column with a flame ionization detector.
Statistical Analysis
Data for growth performance, immune factors, morphological, and VFA analyses are presented as the mean ± SEM and analyzed using SPSS Version 21.0 (SPSS Inc., Chicago, IL). One-way ANOVA and Tukey's multiple comparison tests were performed to evaluate the variation among and between the groups. P < 0.05 indicates statistically significant. Figures were prepared using GraphPad Prism 8.0. Data analysis and figure preparation for the metabolome and microbiome were performed on the Majorbio Cloud Platform as mentioned above.
Correlation assays were conducted between the differential serum metabolites, significant gut microbes (at the genus level), growth indices (BW and ADG), and immune parameters (immunoglobulins and inflammatory cytokines). Data were inputted into SPSS Version 21.0 to calculate the correlation coefficients based on Spearman's correlation distance. Heatmaps were prepared using GraphPad Prism 8.0 to assess bivariate relationships between variables. * P < 0.05, ** P < 0.01, *** P < 0.001.
RESULTS
LA Supplementation Promoted Growth Performance of Broilers
As shown in Table 1, broilers administered ANT and LA showed an increasing trend in BW and ADG during the 42 d feeding period. On d 42, BW was significantly upregulated by ANT and LA treatments (P < 0.05), and the value for the LA1000 group was the highest (2609.74 ± 37.74). As for ADG, ANT treatment performed best in the 1 to 21 d stage, whereas the LA1000 group was best during the 22 to 42 d stage and entire feeding (1–42 d) periods. ADFI in the ANT and LA1000 groups was decreased during 1 to 21 d in comparison to the CON (P < 0.05) but showed no difference during 1 to 42 d (P > 0.05). FCR exhibited a marked decrease when adding ANT and LA during 1 to 21 d (P < 0.05) but did not elicit significant changes thereafter. These results showed that broilers supplemented with LA had similar or better growth performance than those supplemented with ANT, and 1,000 mg/kg was the optimal dosage.
Table 1.
Effect of LA on growth performance of broilers.
| Items | CON | ANT | LA500 | LA1000 | P-value |
|---|---|---|---|---|---|
| BW(g) | |||||
| 1 d | 39.26 ± 0.41 | 39.25 ± 0.49 | 39.08 ± 0.38 | 39.21 ± 0.35 | 0.989 |
| 21 d | 816.22 ± 10.37b | 859.31 ± 14.45ab | 851.93 ± 13.28ab | 877.94 ± 16.35a | 0.028 |
| 42 d | 2451.63 ± 43.68b | 2565.50 ± 35.43a | 2564.13 ± 24.76a | 2609.74 ± 37.74a | 0.028 |
| ADG(g/d) | |||||
| 1–21 d | 26.45 ± 0.52b | 28.07 ± 0.32a | 27.89 ± 0.45ab | 26.87 ± 0.31ab | 0.024 |
| 22–42 d | 86.07 ± 2.06 | 89.34 ± 1.59 | 90.11 ± 1.35 | 91.15 ± 2.25 | 0.263 |
| 1–42 d | 58.84 ± 1.06b | 61.4 ± 0.81ab | 61.59 ± 0.61ab | 62.70 ± 0.92a | 0.027 |
| ADFI | |||||
| 1–21 d | 44.99 ± 0.43a | 41.21 ± 0.67b | 43.31 ± 1.51ab | 40.63 ± 0.67b | 0.008 |
| 22–42 d | 223.21 ± 3.98 | 240.46 ± 4.79 | 244.70 ± 4.03 | 244.36 ± 9.33 | 0.052 |
| 1–42 d | 102.65 ± 1.59b | 105.93 ± 1.50ab | 110.00 ± 1.47a | 107.68 ± 2.24ab | 0.038 |
| FCR | |||||
| 1–21 d | 1.70 ± 0.03a | 1.48 ± 0.02b | 1.56 ± 0.06b | 1.51 ± 0.03b | 0.001 |
| 22–42 d | 1.73 ± 0.04 | 1.79 ± 0.03 | 1.81 ± 0.03 | 1.78 ± 0.09 | 0.734 |
| 1–42 d | 1.75 ± 0.02 | 1.73 ± 0.02 | 1.79 ± 0.02 | 1.72 ± 0.05 | 0.379 |
CON, a basal diet; ANT, a basal diet + 75 mg/kg antibiotic; LA500, a basal diet + 500 mg/kg lauric acid; LA1000, a basal diet + 1000 mg/kg lauric acid; ADG, average daily gain; ADFI, average daily feed intake; BW, body weight; FCR, feed conversion ratio. Data were shown as Mean ± SEM and analyzed by one-way ANOVA Tukey's test. Different letters (a, b, c) in each parameter represent significant (P < 0.05).
LA Improved Intestinal Mucosal Structure of Broilers
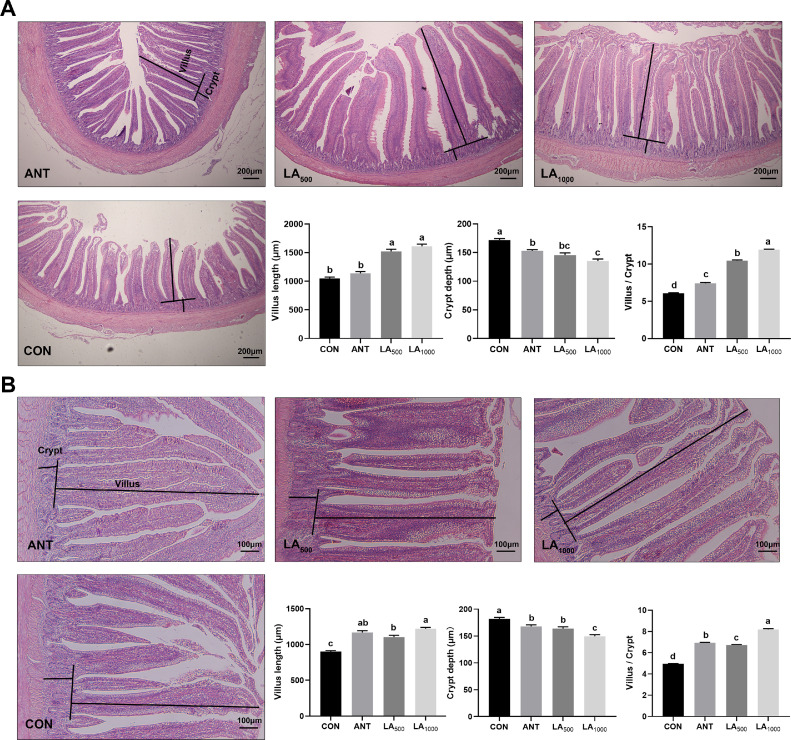
Figure 1 displays the histological structure of the jejunum and ileum on d 42. Compared to the CON group, more complete villi were observed in the LA and ANT groups. As shown in Figure 1A, the villus length of the jejunum was significantly increased in the LA-treated groups (P < 0.05). LA and ANT had dramatically decreased crypt depth (P < 0.05), and a marked reduction occurred in the LA1000 group compared to the ANT group (P < 0.05). The villus/crypt ratio was significantly upregulated by LA and ANT treatments, with the highest was in the LA1000 group. Similar trends were also observed in ileum morphology (Figure 1B). These results indicated that LA could maintain the intestinal structure of broilers, contributing to an enhanced intestinal mucosal barrier.
Figure 1.
Broiler intestinal morphologic analysis by H&E staining. (A) The structure of jejunum, 40 × magnification, scale bar: 200 μm. (B) The structure of ileum, 100 × magnification, scale bar: 100 μm. Villus length and crypt depth were drawn in the images, and calculated by randomly measuring with 10 visual fields of each sample in each group (N = 8). CON: a basal diet; ANT: a basal diet + 75 mg/Kg antibiotic; LA500: a basal diet + 500 mg/Kg lauric acid; LA1000: a basal diet + 1000 mg/Kg lauric acid. Data were shown as Mean ± SEM and analyzed using one-way ANOVA Tukey's test. Different letters (a, b, c, d) in each parameter represent significant (P < 0.05).
LA Enhanced Immune Functions and Inhibited Inflammation of Broilers
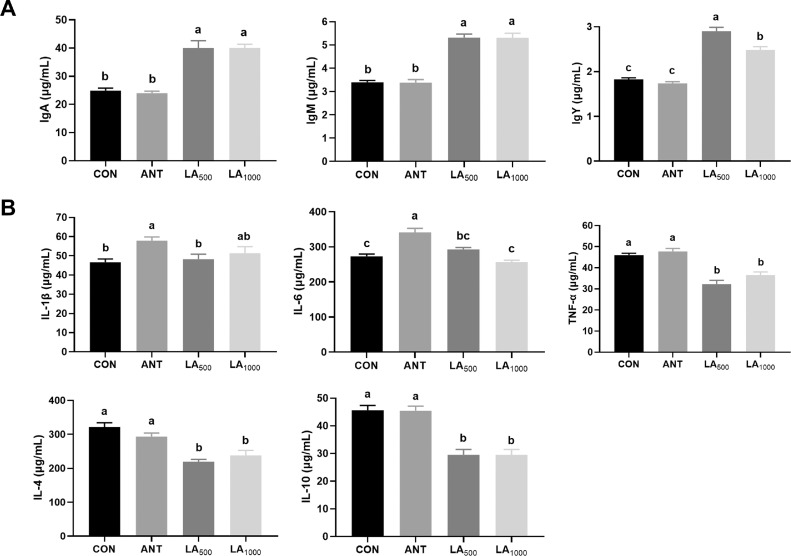
As shown in Figure 2A, broilers fed LA exhibited marked upregulation of the levels of serum immunoglobulins (IgA, IgM, and IgY) (P < 0.05), whereas ANT treatment exhibited no significant difference (P > 0.05). Figure 2B shows the expression of the inflammatory cytokines. Levels of IL-1β and IL-6 was markedly increased in the ANT group (P < 0.05) but exhibited no changes in LA-treated broilers. Compared to the CON and ANT, LA significantly reduced the expression of TNF-α, IL-4, and IL-10 (P < 0.05).
Figure 2.
Effect of LA on the levels of immunoglobulins and inflammatory cytokines in broilers. (A) Concentrations of serum immunoglobulins. (B) Levels of serum inflammatory cytokines. Data were shown as Mean ± SEM and analyzed by one-way ANOVA Tukey's test (N = 8 in each group). Different letters (a, b, c) in each parameter represent significant (P < 0.05).
Multivariate Analysis of Untargeted LC/MS Metabolomics
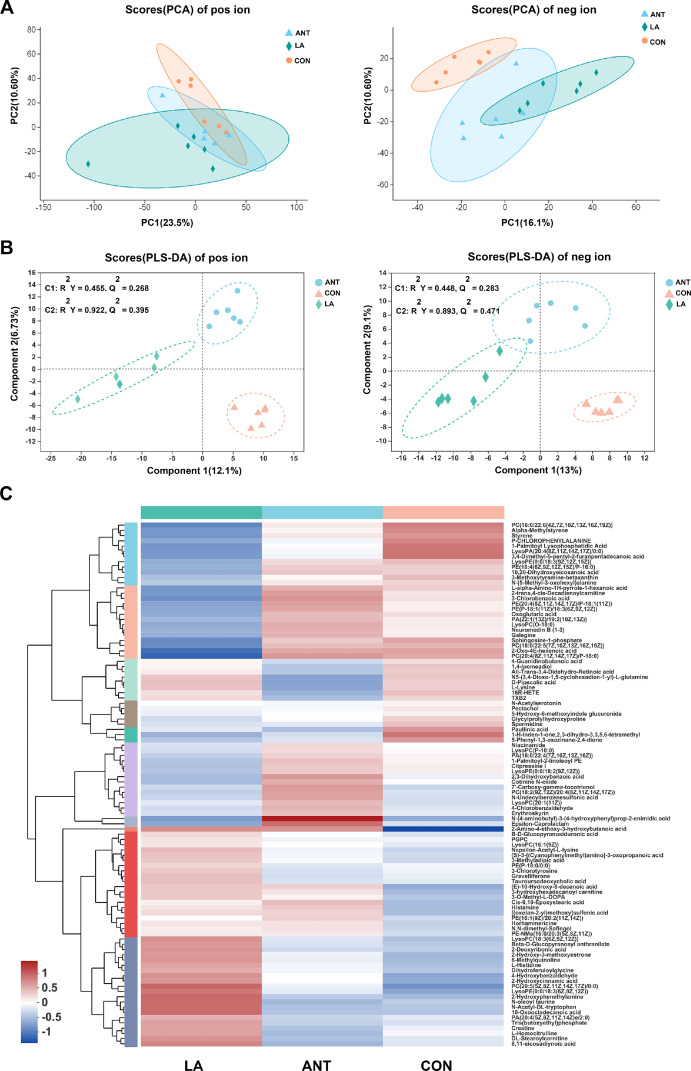
An untargeted HPLC/MS metabolomics analysis was conducted to analyze the differences in serum metabolites. PCA analysis showed that metabolic variation in positive ions were found among the 3 groups (PC1 = 23.5%, PC2 = 10.6%); dots of negative ions in the LA group were separated from the CON and ANT groups (PC1 = 16.1%, PC2 = 10.6%) (Figure 3A). Figure 3B illustrates the PLS-DA analysis. Obvious changes in positive ions were obtained: component 1 = 12.1%, cumulative R2Y at 0.455, Q2 at 0.268; Component 2 = 6.73%, cumulative R2Y at 0.922, Q2 at 0.395. Negative ions in the 3 groups were also completely separated: Component 1 = 13%, cumulative R2Y at 0.448, Q2 at 0.283; Component 2 = 9.1%, cumulative R2Y at 0.893, Q2 at 0.471. The hierarchical clustering heatmap also demonstrated an apparent deviation of LA from CON and ANT (Figure 3C).
Figure 3.
Multivariate analysis of the untargeted LC/MS metabolomics. (A) PCA plots display the variance of positive and negative ions among CON, ANT and LA (1,000 mg/kg) groups (N = 6 per group). (B) PLS-DA score was analyzed by Pareto Scaling method. The plots display the discrimination of positive and negative ions. (C) Heatmap of the hierarchical clustering of Top100 enriched metabolites. Data were analyzed by averaging, hierarchical clustering and Euclidean algorithms. Metabolites were classified with 10 subcluster, and each row represents one metabolite. The shade of colors indicates expression quantity.
LA Modulated Lipid Metabolism of Broilers
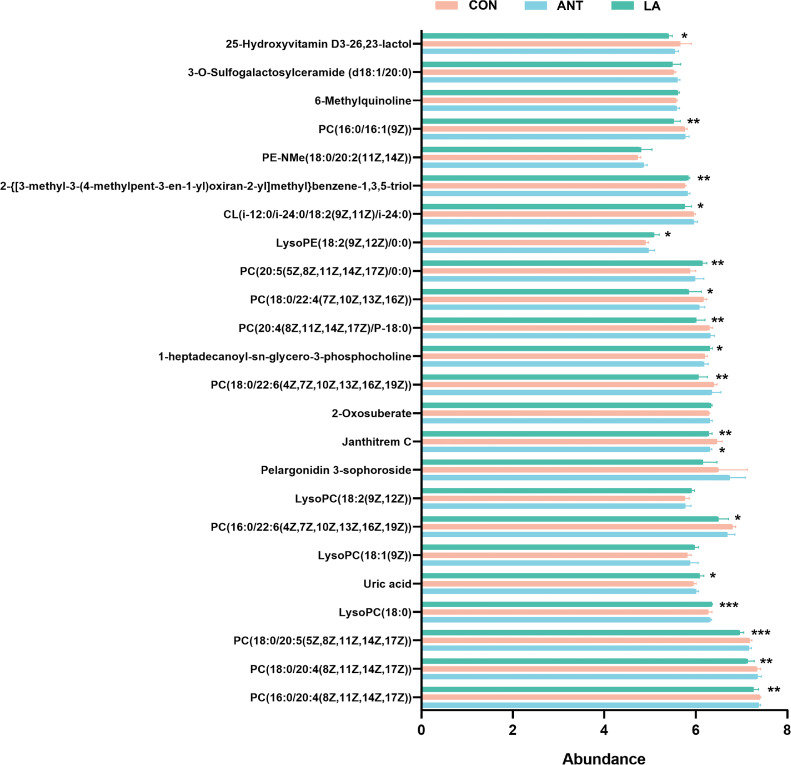
We then targeted the Top100 enriched metabolites to further analyze the metabolic changes. Twenty-four differential metabolites were identified, and LA significantly altered the levels of compounds, primarily the lipids (Figure 4). Specifically, LA markedly downregulated the abundance of phosphatidylcholines (PCs), i.e., PC(16:0/20:4(8Z,11Z,14Z,17Z)), PC(18:0/20: 4(8Z, 11Z,14Z,17Z)), PC(18:0/20:5(5Z, 8Z,11Z,14Z,17Z)), PC(16:0/22:6(4Z,7Z,10Z, 13Z,16Z,19Z)), PC(18:0/22:6 (4Z, 7Z,10Z, 13Z,16Z,19 Z)), PC(20: 4(8Z,11Z,14Z,17Z)/P-18:0), PC(18:0/22:4 (7Z,10Z,13Z,16Z)), and PC(16:0/16:1(9Z)), and increased lysophosphatidylcholine (LysoPC 18:0) and lysophosphatidylethano-lamine(LysoPE 18:2(9Z,12Z) /0:0).
Figure 4.
Analysis of differential metabolites. Statistical analysis of the differential metabolites from the Top100 enriched. Data were shown as Mean ± SEM and analyzed by one-way ANOVA Tukey's test (N = 6 in each group). * represents P < 0.05, ** represents P < 0.01, *** represents P < 0.001.
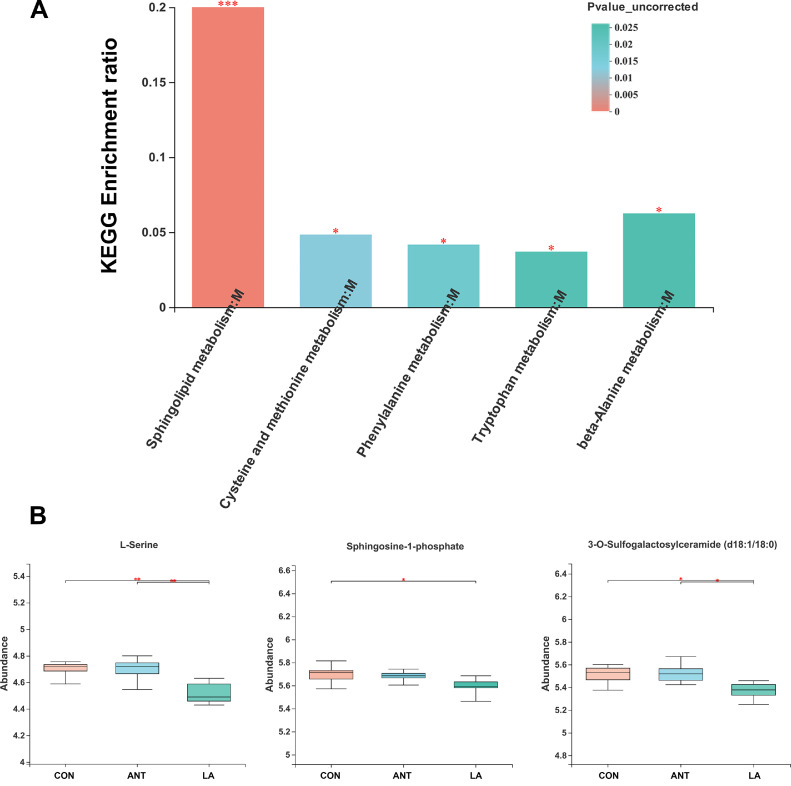
KEGG annotation showed that most compounds belonged to the lipid and amino acid metabolism pathways (FigureS1A). As shown in Figure 5A, sphingolipid metabolism was the dominant pathway (P < 0.001), followed by cysteine and methionine metabolism, phenylalanine metabolism, tryptophan metabolism, and beta-alanine metabolism (P < 0.05). The KEGG pathway map of sphingolipid metabolism revealed that LA significantly downregulated the abundance of L-serine, Sphingosine-1P, and 3-O-sulfogalactosylceramide (d18:1/18:0) (P < 0.01, P < 0.05, P < 0.05, respectively) (Figure S1B and Figure 5B), indicating its role in inhibiting sphingolipid metabolism.
Figure 5.
Analysis of KEGG pathways. (A) Analysis of the enriched significant KEGG pathways. The enriched ratio=metabolite number/background number. (B) The significant metabolites in Sphingolipid metabolism. * represents P < 0.05, ** represents P < 0.01, ***represents P < 0.001.
LA Altered Gut Microbiota Composition of Broilers
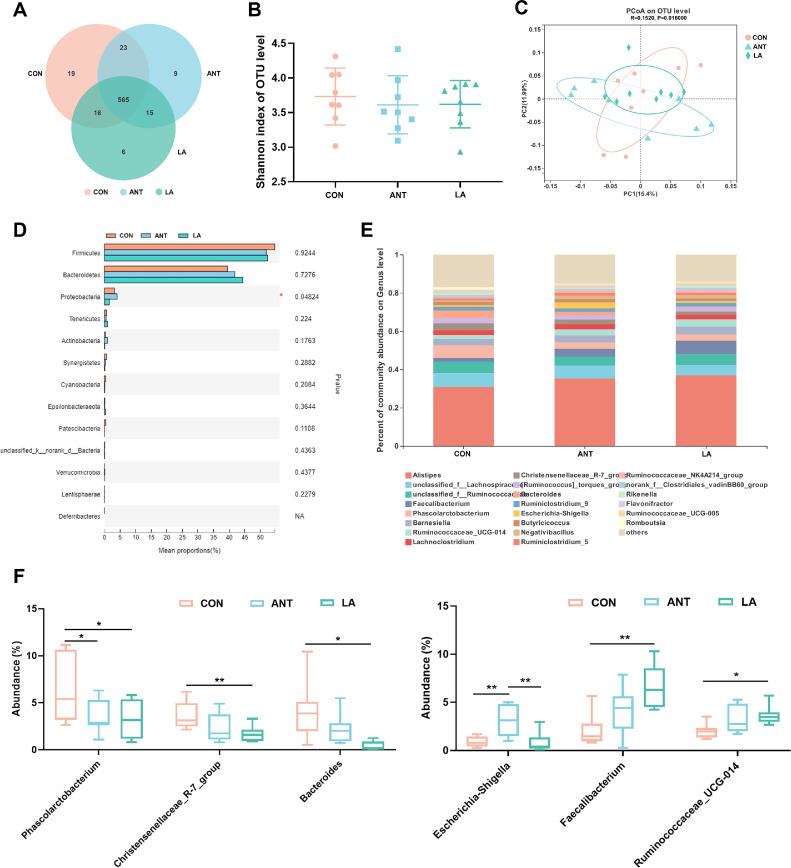
As shown in Figure 6A, the total number of OTUs in the CON, ANT, and LA groups were 623, 612, and 602, respectively. The LA treatment resulted in fewer unique OTUs (6) than the CON (19) and ANT (9) groups. No obvious changes were found in the Shannon index, representing α-diversity (Figure 6B). β-diversity displayed as PCoA scatterplot demonstrated a significant difference in microbiota structure among the 3 groups (ANOSIM r = 0.1520, P = 0.0160), although overlapping clusters were also found (Figure 6C). Alterations in microbiota composition were analyzed at the phylum and genus levels. There were a marked increase in Proteobacteria in the ANT group (4.05%) when compared to the CON (3.30%) and LA (1.54%) groups (P < 0.05) (Figure 6D). Differences at the genus level are shown in Figure 6E and F. Phascolarctobacterium were significantly decreased by LA and ANT treatments (P < 0.05). LA markedly downregulated the richness of the Christensenellaceae_R-7_group and Bacteroides compared to the CON (P < 0.01 and P < 0.05, respectively) but exhibited no significance with ANT. Marked upregulation of Faecalibacterium and Ruminococcaceae_UCG-014 was induced by LA supplementation (P < 0.01 and P < 0.05, respectively). Interestingly, ANT treatment led to a significantly increased proportion of Escherichia-Shigella compared to the CON and LA groups (P < 0.01).
Figure 6.
Analysis of the composition of cecal microbiota. (A) Venn diagram of the total and unique OTUs in each group. (B) The Shannon index reflecting α diversity. (C) β diversity shown as PCoA analysis. The distance algorithm was calculated by unweighted unifrac. (D) Analysis of the significant differences of microbiota composition on phylum level. (E) The microbiota composition on genus level. (F) The significant genera between groups. Data were shown as Mean ± SEM and analyzed by one-way ANOVA Tukey's test (N = 8 in each group). * represents P < 0.05, ** represents P < 0.01.
Analysis of Taxonomic Biomarkers
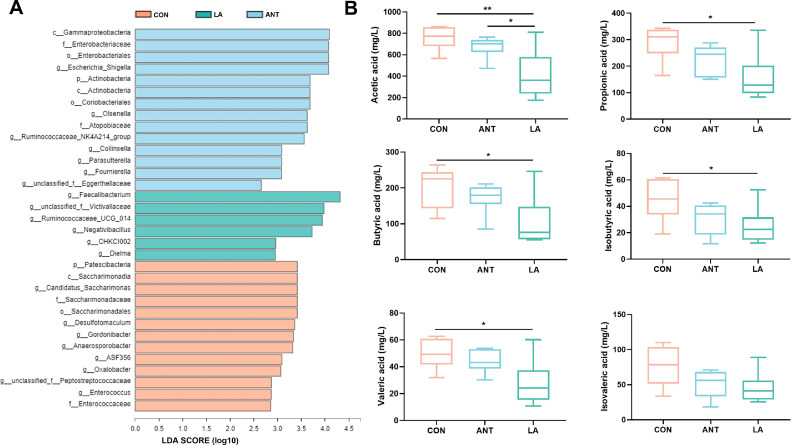
The LEfSe analysis is illustrated in Figure 7A. In the CON group, the predominant bacteria belonged to the same clade: phylum Patescibacteria, class Saccharimonadia, order Saccharimonadales, family Saccharimonadaceae, and genus Candidatus_Saccharimonas (LDA > 3.0, P < 0.01). Taxonomic markers in the LA group included the genera Faecalibacterium (LDA = 4.32, P < 0.05), unclassified_f_Vactivallaceae (LDA=3.98, P < 0.05), Ruminococcaceae_ UCG-014 (LDA = 3.94, P < 0.05), Negativibacillus, CHKCI002 (LDA=2.96, P < 0.01), and Dielma (LDA=2.95, P < 0.05). ANT treatment significantly increased the abundance of the Proteobacteria phylum (including c_Gammaproteobacteria, f_Enterobacteriaceae, and g_Escherichia_ shigella, LDA > 3.5, P < 0.05), and the phylum Actinobacteria (including c_ Actinobacteria, o_Coriobacteriales, f_ Atopobiaceae, and g_Olsenella, LDA > 3.0, P < 0.05). These results suggest that significant microbial variations exist among the groups.
Figure 7.
Analysis of LefSE and VFA production. (A) Histogram of LDA scores for taxonomic biomarkers identified by LEfSe. LDA score (log10) >2 indicates enriched taxa in cases. (B) Cecal VFA production. Data were shown as Mean ± SEM analyzed by one-way ANOVA Tukey's test (N = 8 in each group). * represents P < 0.05, ** represents P < 0.01.
LA Downregulated VFA Production of Broilers
As displayed in Figure 7B, broilers treated with LA showed a downward trend in cecal VFA production. Specifically, LA significantly decreased acetic acid and propionic acid levels compared to that in the CON group (P < 0.01 and P < 0.05, respectively). The concentrations of butyrate acid, isobutyric acid, and valerate acid were also significantly decreased by LA treatment (P < 0.05). No significant differences were found in isovalerate acid among the 3 groups (P > 0.05).
Spearman Correlation Analysis
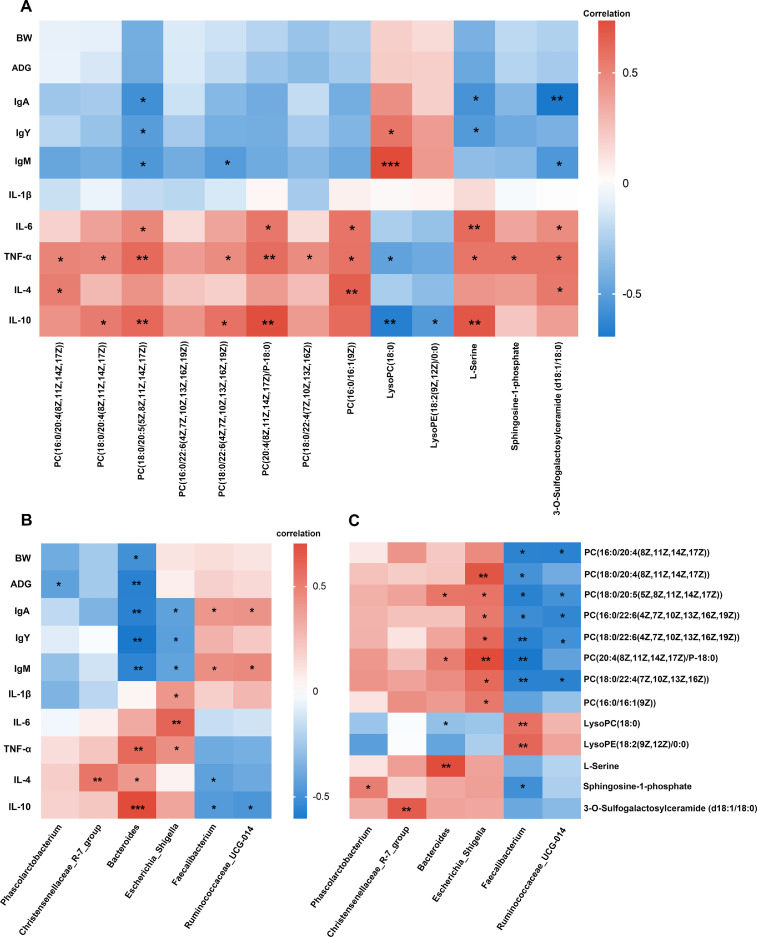
Finally, we assessed the relationships between the differential metabolites, gut microbes, growth, and immune parameters using Spearman's correlation analysis. As shown in Figure 8A, the PCs were highly positively correlated with the cytokines, particularly IL-6, TNF-α, and IL-10, and negatively correlated with immunoglobulins, whereas LysoPC exhibited a reverse relationship. The 3 significant metabolites belonging to the sphingolipid metabolism pathway were positively correlated with cytokines and negatively correlated with immunoglobulins. Figure 8B shows the correlation between growth and immune parameters and gut microbes. An obvious negative relationship was found between Phascolarctobacterium and ADG. Bacteroides had the highest correlations with most indices, a significant negative correlation with BW, ADG, SIgA, IgM, and IgY, and a marked positive correlation with TNF-α, IL-4, and IL-10. Escherichia_Shigella was significantly negatively correlated with immunoglobulins and positively correlated with cytokines, whereas Faecalibacterium and Ruminococcaceae_UCG014 exhibited the opposite trend. Figure 8C shows the correlation between the differential genera and serum metabolites. Escherichia_Shigella and Faecalibacterium were highly correlated with the metabolites. Escherichia_Shigella was highly positively correlated with almost all PCs. Faecalibacterium exhibited a marked negative relationship with PCs and Sphingosine-1P and a positive relationship with LysoPC and LysoPE. Ruminococcaceae_UCG-014 showed a negative relationship with PCs, whereas Bacteroides exhibited the opposite trend.
Figure 8.
Heatmaps of Spearman's correlation analysis. (A) Correlation of differential metabolites with growth and immune indices. (B) Correlation of gut microbiota with growth and immune indices. (C) Correlation between gut microbiota and differential metabolites. * P < 0.05, ** P < 0.01, *** P < 0.001.
DISCUSSION
Due to the growing concerns regarding antibiotic residues on food safety, research currently has been focused on developing substitutes to meet “Raised Without Antibiotics” requirements in poultry production. In the present study, LA supplementation significantly promoted the growth performance of broilers, which was even better than that of aureomycin, indicating its potential to replace antibiotics. Moreover, LA markedly increased the intestinal villus length and V/C ratio. Longer villi indicate a greater absorption surface, whereas the V/C ratio reflects more robust intestinal functions (Chen et al., 2013; Hafez et al., 2017). It was reported that LA could be used as an effective absorption enhancer; LA prevented intestinal barrier dysfunction and enhanced mucosal structure in mice challenged with pathogens (Ghadiri et al., 2019). Consistent with previous studies, our results revealed the advantages of LA in improving intestinal absorptive capacity and maintaining the mucosal barrier.
LA has previously been shown to possess immunoregulatory functions in many studies. For example, coconut oil (LA-enriched) could regulate IgG and IgA concentrations in the plasma of sows (Bai et al., 2017). A mix of LA and other organic acids significantly increased serum IgM and IgG levels in weaned piglets (Han et al., 2020). Similarly, we found that LA significantly upregulated the concentrations of serum IgA, IgM, and IgY in broilers. Furthermore, LA is well known for its antibacterial and anti-inflammatory properties. It acts as an inhibitor of various pathogens, such as Clostridium difficile, Escherichia coli, and Campylobacter, and can significantly reduce inflammation during infectious diseases (Zeiger et al., 2017; Yang et al., 2018; Jin et al., 2021). In the present study, LA markedly ameliorated serum IL-6, TNF-α, IL-4, and IL-10 levels. Inflammatory cytokines are generally believed to be rapidly released during infection or other stimuli, whereas they remain at basal levels under physiological conditions (Kany et al., 2019). In our study, broilers were raised under normal conditions, and the inflammatory responses were not highly activated. The lower levels of inflammatory cytokines induced by LA treatment further indicated its ability to suppress inflammatory responses.
Our results showed that LA modulated lipid metabolism by reducing the abundance of PCs and regulating the sphingolipid metabolism pathway. PCs are the most enriched phospholipid class in the serum and represent an important biomarker for evaluating lipid absorption and metabolism (Pacetti et al., 2004). They are normally made from choline via the CDP-choline pathway, secreted into bile, and required for lipoprotein assembly and secretion (Li, 2008; Rey et al., 2011). Lower PCs are believed to exert dual functions in inflammation. It has been reported that PCs promote adipose tissue inflammation in obesity (Petkevicius et al., 2019), whereas polyene PC inhibits the inflammatory response in LPS-stimulated macrophages and ameliorates rat arthritis (Pan et al., 2017). We found that LA significantly decreased the abundance of PCs, and PCs were positively correlated with inflammatory cytokines, which were downregulated by LA treatment. Our results indicated that LA might alleviate inflammation by inhibiting PCs. Moreover, LA markedly increased the LysoPC abundance. LysoPCs are a class of lipid biomolecules derived from the cleavage of PCs and have a direct role in alleviating systemic inflammatory disorders (Cunningham et al., 2008). Accordingly, our study showed that LysoPC was negatively correlated with inflammatory cytokines and positively correlated with IgM and IgY, which further suggests the protective role of LA in inhibiting inflammation and enhancing immunity. KEGG analysis showed that LA could modulate sphingolipid metabolism by decreasing the levels of the initial substrate L-serine and the major metabolic product Sphingosine-1P (S1P). Sphingolipid metabolism affects many cellular processes, including apoptosis, proliferation, and senescence (Gault et al., 2010). It has been reported that S1P participates in regulating cellular processes, normally remains at a low level, and has been considered a tumor-promoting lipid (Le Stunff et al., 2002; Oskouian and Saba, 2010). Thus, decreased S1P by LA treatment reflected the optimal physiological status of broilers, which is consistent with the view that external stimuli activate S1P production and eventually promote cell apoptosis (Wu et al., 2017). In brief, we demonstrated that LA could modulate lipid metabolism, contributing to enhanced immune functions and inhibited inflammation in broilers.
We further demonstrated that LA has a strong ability to alter the gut microbiota in broilers. It decreased the total OTUs compared to CON and ANT, which was probably caused by its strong antibacterial capacity. LA has been reported to inhibit bacterial growth by creating an acidic environment and disintegrating bacterial cell walls (Anzaku et al., 2017). Accordingly, the decreased microbiota might explain the downregulation of VFA production with LA treatment. Previous studies support these results. For example, feeding LA or coconut oil markedly decreased the total VFAs in dairy cows (Faciola et al., 2014), and LA dramatically affected ruminal fermentation because of decreased VFAs (Hristov et al., 2011). Furthermore, our study showed that LA significantly changed the microbial composition by decreasing the abundance of Phascolarctobacterium, Christensenellaceae_ R-7_group, and Bacteroides, and increasing Faecalibacterium and Ruminococcaceae_UCG-014. Phascolarctobacterium is a genus that produces VFAs, including acetate and propionate (Wu et al., 2017). The decrease in Phascolarctobacterium further elucidated the cause of VFA declined by LA treatment. Christensenellaceae_R-7_group affects host BW and is enriched in individuals with a low body mass index and negatively associated with ADG (Goodrich et al., 2014; Fang et al., 2020). We observed a slight negative correlation of this genus with BW and ADG, but the correlation was not significant. Bacteroides is commonly considered to maintain a complex and beneficial relationship with the host, including fermentation of carbohydrates to produce VFAs as an energy source for host utilization and is positively correlated with growth performance (Zhu et al., 2021). However, our study showed an opposite trend: Bacteroides was highly negatively correlated with growth parameters, and LA significantly reduced its abundance. Although Bacteroides plays an important role in promoting host health, its species have also been implicated in many diseases. B. fragilis has been shown to generate endotoxins and plays a role in driving proinflammation (Lukiw, 2016; Chung et al., 2018); B. thetaiotaomicron and B. vulgatus were found to enhance the pathogenicity and expression of virulence genes in E. coli (Wexler et al., 2017). Our results revealed that Bacteroides was negatively correlated with immunoglobulins and positively correlated with inflammatory cytokines. Thus, in our study, decreased Bacteroides with LA treatment positively affected immune functions. Faecalibacterium was markedly upregulated in the LA group. This genus is crucial for gut homeostasis and is considered a protective factor for the gut mucosa (Sokol et al., 2009). The well-known species F. prausnitzii has been shown to exhibit immunomodulatory and anti-inflammatory properties in many studies (Quévrain et al., 2016; Lopez-Siles et al., 2017). We found that this genus was positively correlated with immunoglobulins and LysoPE and negatively correlated with PCs; thus, the increased levels with LA treatment indicated its ability to enhance immunity. Consistent with our results, broilers fed dietary vitamins harbored a higher ratio of Faecalibacterium, contributing to the promotion of gut health (Luo et al., 2013). The increased abundance of Ruminococcaceae_UCG-014 in LA treatment suggests an improved microbiota because this genus is a stable microbial component of the cecum and plays a critical role in fiber degradation (Gaukroger et al., 2020). Additionally, the above 3 genera were highly negatively correlated with PCs and positively correlated with immunoglobulins, suggesting that their increase might contribute to modulating lipid metabolism and immunity of broilers. Taken together, we speculate that the microbiota altered by LA was the key factor in enhancing immune functions, suppressing inflammation, and modulating lipid metabolism, which then eventually improved the growth performance and health of broilers.
Notably, the phylum Proteobacteria and its genus Escherichia-Shigella were significantly increased in the ANT treatment, and these results were further confirmed by LefSE analysis. In our study, as a growth promoter supplemented in an animal diet, the antibiotic was applied at a subtherapeutic dosage and continuously fed to the chickens for a long period. These conditions might generate antibiotic-resistant bacteria. Concerns about the development of bacterial resistance with IFA supplementation have long been considered and ultimately lead to prohibitive legislation/guidance by many countries (Broom et al., 2017). Furthermore, antibiotics are believed to disturb the stability and balance of the gut ecosystem, thereby facilitating pathogen invasion and colonization (Rivera-Chávez et al., 2016). Therefore, it is possible that supplementation with antibiotics increased the abundance of Escherichia-Shigella. Consistent with our findings, previous studies have shown that the percentages of Proteobacteria and Escherichia-Shigella were higher after antibiotic treatment (Li et al., 2019; Tian et al., 2020). A high proportion of Proteobacteria and Escherichia-Shigella reflects gut dysbiosis and is associated with many diseases, such as diarrhea, inflammatory bowel diseases, and pathogenesis of colitis (Shin et al., 2015; Sun et al., 2019; Zhang et al., 2020). In our study, Escherichia-Shigella was positively correlated with the proinflammatory cytokines (IL-1β, IL-6, and TNF-α), whereas ANT significantly upregulated the levels of IL-1β and IL-6. It was reported that the increase of Escherichia-Shigella could lead to upregulated inflammatory responses of broilers (Liu et al., 2020). Thus, the increased levels of proinflammatory cytokines might be caused by the increased Escherichia-Shigella. Previous studies have demonstrated that antibiotic treatment can increase the inflammatory response. For instance, early antibiotic intervention significantly increased gene expression of proinflammatory cytokines (IFN-γ and IL-8) in a piglet model (Zhang et al., 2017).
In summary, we found that LA promoted growth performance, enhanced immune functions, inhibited inflammation, modulated lipid metabolism, and altered the cecal microbial composition in broilers. Our findings highlight the substantial potential of LA as an antibiotic substitute in poultry diets.
ACKNOWLEDGMENTS
This study was supported by Zhejiang Provincial Key Research and Development Program (No. 2017C02005 and No.2019C02051), the National Key Research and Development Program of China (2018YFE0112700), the Natural Science Foundation of Zhejiang Province (No. LQ21C170001 and No. LQ20C170003), the Science and Technology Innovative Program for college students of Zhejiang Province (new-shoot Talents Program) (Grants No.2020R412040), and the Scientific Research Project of Zhejiang Provincial Education Department (No.YZ20200001 and No.YZ20200002).
Author contributions: The authors’ contributions are as follows: C. Y. designed and supervised the study; Y. p. W. drafted the manuscript; C. Y., Y. x. W., G. C. and H. Z. revised the manuscript; H. Z., R. Z., Q. L. and B., Z. conducted the experiments; Y. p. W. performed data analysis. All authors have read and approved the final version of the manuscript.
DISCLOSURES
The authors declare no financial or personal conflicts of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psj.2021.101315.
Appendix. Supplementary materials
REFERENCES
- Agus A., Planchais J., Sokol H. Gut Microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23:716–724. doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- Anzaku A.A., Akyala J.I., Juliet A., Obianuju E.C. Antibacterial activity of lauric acid on some selected clinical isolates. Ann. Clin. Res. 2017;5:170–174. [Google Scholar]
- Arias V.J., Koutsos E.A. Effects of copper source and level on intestinal physiology and growth of broiler chickens. Poult. Sci. 2006;85:999–1007. doi: 10.1093/ps/85.6.999. [DOI] [PubMed] [Google Scholar]
- Bai Y.S., Wang C.Q., Zhao X., Shi B.M., Shan A.S. Effects of fat sources in sow on the fatty acid profiles and fat globule size of milk and immunoglobulins of sows and piglets. Anim. Feed Sci. Tech. 2017;234:217–227. [Google Scholar]
- Broom L.J. The sub-inhibitory theory for antibiotic growth promoters. Poult. Sci. 2017;96:3104–3108. doi: 10.3382/ps/pex114. [DOI] [PubMed] [Google Scholar]
- Chen Z., Tang J., Sun Y.Q., Xie J. Protective effect of gamma-aminobutyric acid on antioxidation function in intestinal mucosa of Wenchang chicken induced by heat stress. J. Anim. Plant Sci. 2013;23:1634–1641. [Google Scholar]
- Cheng G., Hao H., Xie S., Wang X., Dai M., Huang L., Yuan Z.H. Antibiotic alternatives: the substitution of antibiotics in animal husbandry? Front. Microbiol. 2014;5:217–232. doi: 10.3389/fmicb.2014.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung L., Orberg E.T., Geis A.L., Chan J.L., Fu K., DeStefano Shields C.E., Dejea C.M., Fathi P., Chen J., Finard B.B., Tam A.J., McAllister F., Fan H., Wu X., Ganguly S., Lebid A., Metz P., Van Meerbeke S.W., Huso D.L., Wick E.C., Pardoll D.M., Wan F., Wu S., Sears C.L., Housseau F. Bacteroides fragilis Toxin Coordinates a Pro-carcinogenic Inflammatory Cascade via Targeting of Colonic Epithelial Cells. Cell Host. Microbe. 2018;23:421. doi: 10.1016/j.chom.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham T.J., Yao L., Lucena A. Product inhibition of secreted phospholipase A2 may explain lysophosphatidylcholines' unexpected therapeutic properties. J. Inflamm.-Lond. 2008;5:17–26. doi: 10.1186/1476-9255-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahiya J.P., Wilkie D.C., Van Kessel A.G., Drew M.D. Potential strategies for controlling necrotic enteritis in broiler chickens in post-antibiotic era. Anim. Feed Sci. Tech. 2006;129:60–88. [Google Scholar]
- Dayrit F.M. The properties of lauric acid and their significance in coconut oil. J. Am. Oil Chem. Soc. 2015;92:1–15. [Google Scholar]
- Faciola A.P., Broderick G.A. Effects of feeding lauric acid or coconut oil on ruminal protozoa numbers, fermentation pattern, digestion, omasal nutrient flow, and milk production in dairy cows. J. Dairy Sci. 2014;97:5088–5100. doi: 10.3168/jds.2013-7653. [DOI] [PubMed] [Google Scholar]
- Fang S.M., Chen X., Pan J.H., Chen Q.H., Zhou L.W., Wang C.C., Xiao T.F., Gan Q.F. Dynamic distribution of gut microbiota in meat rabbits at different growth stages and relationship with average daily gain (ADG) Bmc Microbio. 2020;20:116–129. doi: 10.1186/s12866-020-01797-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint H.J., Scott K.P., Louis P., Duncan S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012;9:577–589. doi: 10.1038/nrgastro.2012.156. [DOI] [PubMed] [Google Scholar]
- Gaukroger C.H., Stewart C.J., Edwards S.A., Walshaw J., Adams I.P., Kyriazakis I. Changes in faecal microbiota profiles associated with performance and birthweight of piglets. Front. Microbiol. 2020;11:917–930. doi: 10.3389/fmicb.2020.00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gault C.R., Obeid L.M., Hannun Y.A. An overview of sphingolipid metabolism: from synthesis to breakdown. Adv. Exp. Med. Biol. 2010;688:1–23. doi: 10.1007/978-1-4419-6741-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadiri M., Young P.M., Traini D. Strategies to Enhance Drug Absorption via Nasal and Pulmonary Routes. Pharmaceutics. 2019;11:113–132. doi: 10.3390/pharmaceutics11030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich J.K., Waters J.L., Poole A.C., Sutter J.L., Koren O., Blekhman R., Beaumont M., Van Treuren W., Knight R., Bell J.T., Spector T.D., Clark A.G., Ley R.E. Human genetics shape the gut microbiome. Cell. 2014;159:789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafez A., Hegazi S., Bakr A. Effect of zinc oxide nanoparticles on growth performance and absorptive capacity of the intestinal villi in broiler chickens. Life. Sci. J. 2017;14:67–72. [Google Scholar]
- Han Y., Zhan T., Zhao Q., Tang C., Zhang K., Han Y., Zhang J. Effects of mixed organic acids and medium chain fatty acids as antibiotic alternatives on the performance, serum immunity, and intestinal health of weaned piglets orally challenged with escherichia coli k88. Anim. Feed Sci. Tech. 2020;269:114617–114661. [Google Scholar]
- Hanczakowska E. The use if medium-chain fatty acids in piglet feeding-a review. Ann. Anim. Sci. 2017;17:967–977. [Google Scholar]
- Hristov A.N., Lee C., Cassidy T., Long M., Heyler K., Corl B., Forster R. Effects of lauric and myristic acids on ruminal fermentation, production, and milk fatty acid composition in lactating dairy cows. J Dairy Sci. 2011;94:382–395. doi: 10.3168/jds.2010-3508. [DOI] [PubMed] [Google Scholar]
- Jin X., Zhou J., Richey G., Wang M., Hong S.M.C., Hong S.H. Undecanoic Acid, Lauric Acid, and N-Tridecanoic Acid Inhibit Escherichia coli Persistence and Biofilm Formation. J. Microbiol. Biotechnol. 2021;31:130–136. doi: 10.4014/jmb.2008.08027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kany S., Vollrath J.T., Relja B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019;20:6008. doi: 10.3390/ijms20236008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C., Browne W.J., Cuthill I.C., Emerson M., Altman D.G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Osteoarthritis. Cartilage. 2012;20:256–260. doi: 10.1016/j.joca.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Lappano R., Sebastiani A., Cirillo F., Rigiracciolo D.C., Galli G.R., Curcio R., Malaguarnera R., Belfiore A., Cappello A.R., Maggiolini M. The lauric acid-activated signaling prompts apoptosis in cancer cells. Cell Death Discov. 2017;3:17063–17071. doi: 10.1038/cddiscovery.2017.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Stunff H., Galve-Roperh I., Peterson C., Milstien S., Spiegel S. Sphingosine-1-phosphate phosphohydrolase in regulation of sphingolipid metabolism and apoptosis. J. Cell. Biol. 2002;158:1039–1049. doi: 10.1083/jcb.200203123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Xiao B., Zhang Y., Xiao S., Luo J., Huang W. Impact of maternal intrapartum antibiotics on the initial oral microbiome of neonates. Pediatr. Neonatol. 2019;60:654–661. doi: 10.1016/j.pedneo.2019.03.011. [DOI] [PubMed] [Google Scholar]
- Li Z., Vance D.E. Thematic review series: glycerolipids. Phosphatidylcholine and choline homeostasis. J. Lipid. Res. 2008;49:1187–1194. doi: 10.1194/jlr.R700019-JLR200. [DOI] [PubMed] [Google Scholar]
- Liu Q.X., Zhou Y., Li X.M., Ma D.D., Xing S., Feng J.H., Zhang M.H. Ammonia induce lung tissue injury in broilers by activating NLRP3 inflammasome via Escherichia/Shigella. Poult. Sci. 2020;99:3402–3410. doi: 10.1016/j.psj.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Li C., Li Y., Feng F. Glycerol monolaurate enhances reproductive performance, egg quality and albumen amino acids composition in aged hens with gut microbiota alternation. Agriculture. 2020;10:250–263. [Google Scholar]
- Lopez-Siles M., Duncan S.H., Garcia-Gil L.J., Martinez-Medina M. Faecalibacterium prausnitzii: from microbiology to diagnostics and prognostics. Isme. J. 2017;11:841–852. doi: 10.1038/ismej.2016.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukiw W.J. Bacteroides fragilis lipopolysaccharide and inflammatory signaling in Alzheimer's Disease. Front. Microbiol. 2016;7:1544–1550. doi: 10.3389/fmicb.2016.01544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y.H., Peng H.W., Wright A.D.G., Bai S.P., Ding X.M., Zeng Q.F. Broilers fed dietary vitamins harbor higher diversity of cecal bacteria and higher ratio of Clostridium, Faecalibacterium, and Lactobacillus than broilers with no dietary vitamins revealed by 16S rRNA gene clone libraries. Poult. Sci. 2013;92:2358–2366. doi: 10.3382/ps.2012-02935. [DOI] [PubMed] [Google Scholar]
- Maier L., Pruteanu M., Kuhn M., Zeller G., Telzerow A., Anderson E.E., Brochado A.R., Fernandez K.C., Dose H., Mori H., Patil K.R., Bork P., Typas A. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555:623–628. doi: 10.1038/nature25979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsue M., Mori Y., Nagase S., Sugiyama Y., Hirano R., Ogai K., Ogura K., Kurihara S., Okamoto S. Measuring the antimicrobial activity of lauric acid against various bacteria in human gut microbiota using a new method. Cell Transplant. 2019;28:1528–1541. doi: 10.1177/0963689719881366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskouian B., Saba J.D. Cancer treatment strategies targeting sphingolipid metabolism. Adv. Exp. Med. Biol. 2010;688:185–205. doi: 10.1007/978-1-4419-6741-1_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omonijo F.A., Kim S., Guo T., Wang Q., Gong J., Lahaye L., Bodin J.C., Nyachoti M., Liu S., Yang C. Development of novel microparticles for effective delivery of thymol and lauric acid to pig intestinal tract. J. Agric. Food Chem. 2018;66:9608–9615. doi: 10.1021/acs.jafc.8b02808. [DOI] [PubMed] [Google Scholar]
- Pacetti D., Malavolta M., Bocci F., Boselli E., Frega N.G. High-performance liquid chromatography/electrospray ionization ion-trap tandem mass spectrometric analysis and quantification of phosphatidylcholine molecular species in the serum of cystic fibrosis subjects supplemented with docosahexaenoic acid. Rapid Commun. Mass Spectrom. 2004;18:2395–2400. doi: 10.1002/rcm.1639. [DOI] [PubMed] [Google Scholar]
- Pan W., Hao W.T., Xu H.W., Qin S.P., Li X.Y., Liu X.M., Sun F.F., Li H., Tang R.X., Zheng K.Y. Polyene Phosphatidylcholine inhibited the inflammatory response in LPS-stimulated macrophages and ameliorated the adjuvant-induced rat arthritis. Am J Transl Res. 2017;9:4206–4216. [PMC free article] [PubMed] [Google Scholar]
- Petkevicius K., Virtue S., Bidault G., Jenkins B., Cubuk C., Morgantini C., Aouadi M., Dopazo J., Serlie M.J., Koulman A., Vidal-Puig A. Accelerated phosphatidylcholine turnover in macrophages promotes adipose tissue inflammation in obesity. Elife. 2019;8:e47990. doi: 10.7554/eLife.47990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevrain E., Maubert M.A., Michon C., Chain F., Marquant R., Tailhades J., Miquel S., Carlier L., Bermudez-Humaran L.G., Pigneur B., Lequin O., Kharrat P., Thomas G., Rainteau D., Aubry C., Breyner N., Afonso C., Lavielle S., Grill J.P., Chassaing G., Chatel J.M., Trugnan G., Xavier R., Langella P., Sokol H., Seksik P. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn's disease. Gut. 2016;65:415–425. doi: 10.1136/gutjnl-2014-307649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey J.W., Schreiner O., Barreiros A.P., Heise M., Krupp M., Schuchmann M., Otto G., Galle P.R., Teufel A. Acute renal failure and liver dysfunction after subcutaneous injection of 3-sn-phosphatidylcholine (Lipostabil(R))-case report. Z. Gastroenterol. 2011;49:340–343. doi: 10.1055/s-0029-1245614. [DOI] [PubMed] [Google Scholar]
- Rivera-Chavez F., Zhang L.F., Faber F., Lopez C.A., Byndloss M.X., Olsan E.E., Xu G., Velazquez E.M., Lebrilla C.B., Winter S.E., Baumler A.J. Depletion of butyrate-producing clostridia from the gut microbiota drives an aerobic luminal expansion of salmonella. Cell Host. Microbe. 2016;19:443–454. doi: 10.1016/j.chom.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland I., Gibson G., Heinken A., Scott K., Swann J., Thiele I., Tuohy K. Gut microbiota functions: metabolism of nutrients and other food components. Eur. J. Nutr. 2018;57:1–24. doi: 10.1007/s00394-017-1445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y., Wen Q., Zhang S., Lu L., Mang L., Liao X., Luo X. Dietary supplemental vitamin D-3 enhances phosphorus absorption and utilisation by regulating gene expression of related phosphate transporters in the small intestine of broilers. Br. J. Nutr. 2019;121:9–21. doi: 10.1017/S0007114518002763. [DOI] [PubMed] [Google Scholar]
- Shin N.R., Whon T.W., Bae J.W. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33:496–503. doi: 10.1016/j.tibtech.2015.06.011. [DOI] [PubMed] [Google Scholar]
- Simo-Mirabet P., Piazzon M.C., Calduch-Giner J.A., Ortiz A., Puyalto M., Sitja-Bobadilla A., Perez-Sanchez J. Sodium salt medium-chain fatty acids and Bacillus-based probiotic strategies to improve growth and intestinal health of gilthead sea bream (Sparus aurata) PeerJ. 2017;5:e4001. doi: 10.7717/peerj.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol H., Seksik P., Furet J.P., Firmesse O., Nion-Larmurier I., Beaugerie L., Cosnes J., Corthier G., Marteau P., Dore J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm. Bowel. Dis. 2009;15:1183–1189. doi: 10.1002/ibd.20903. [DOI] [PubMed] [Google Scholar]
- Sol C., Puyalto M., Lindenbeck M., Mallo J.J., Zentek J. PSIX-36 Effect of lauric acid based additives on piglets fecal microbiota. J. Anim. Sci. 2019;97(Suppl 3):352. [Google Scholar]
- Sun X., Gao Y., Wang X., Hu G., Wang Y., Feng B. Escherichia coli O-101-induced diarrhea develops gut microbial dysbiosis in rats. Ex. The. Me. 2019;17:824–834. doi: 10.3892/etm.2018.6997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanh N.T., Loh T.C., Foo H.L., Hair-Bejo M., Azhar B.K. Effects of feeding metabolite combinations produced by Lactobacillus plantarum on growth performance, faecal microbial population, small intestine villus height and faecal volatile fatty acids in broilers. Br. Poult. Sci. 2009;50:298–306. doi: 10.1080/00071660902873947. [DOI] [PubMed] [Google Scholar]
- Tian B.M., Liu M.Y., An W., Yu L.Z., Zhang J.W., Liu Y.L. Lycium barbarum relieves gut microbiota dysbiosis and improves colonic barrier function in mice following antibiotic perturbation. J. Funct. Foods. 2020;71:103973–119985. [Google Scholar]
- Wexler A.G., Goodman A.L. An insider's perspective: Bacteroides as a window into the microbiome. Nat. Microbiol. 2017;2(5):1–11. doi: 10.1038/nmicrobiol.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Guo X., Zhang J., Zhang M., Ou Z., Peng Y. Phascolarctobacteriumáfaecium abundant colonization in human gastrointestinal tract. Exp. Ther. Med. 2017;14:3122–3126. doi: 10.3892/etm.2017.4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Zhang L., Cao G., Feng J., Yue M., Xu Y., Dai B., Han Q., Guo X. Effects of dietary supplementation with essential oils and organic acids on the growth performance, immune system, fecal volatile fatty acids, and microflora community in weaned piglets. J. Anim. Sci. 2019;97:133–143. doi: 10.1093/jas/sky426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W.Y., Lee Y., Lu H., Chou C.H., Wang C. Analysis of gut microbiota and the effect of lauric acid against necrotic enteritis in Clostridium perfringens and Eimeria side-by-side challenge model. PLoS One. 2019;14 doi: 10.1371/journal.pone.0205784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Chen J., Rathod J., Jiang Y., Tsai P., Hung Y., Ko W., Paredes-Sabja D., Huang I. Lauric acid is an inhibitor of clostridium difficile growth in vitro and reduces inflammation in a mouse infection model. Front. Microbiol. 2018;8:2635–2650. doi: 10.3389/fmicb.2017.02635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger K., J Popp, Becker A., Hankel J., Visscher C., Klein G., Meemken D. Lauric acid as feed additive–An approach to reducing Campylobacter spp. in broiler meat. PloS One. 2017;12 doi: 10.1371/journal.pone.0175693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Yu M., Yang Y., Mu C., Su Y., Zhu W. Differential effect of early antibiotic intervention on bacterial fermentation patterns and mucosal gene expression in the colon of pigs under diets with different protein levels. Appl. Microb. Biotech. 2017;101:2493–2505. doi: 10.1007/s00253-016-7985-7. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Taylor L., Shommu N., Ghosh S., Reimer R., Panaccione R., Raman M. A diversified dietary pattern is associated with a balanced gut microbial composition of Faecalibacterium and Escherichia/Shigella in patients with Crohn's disease in remission. J. Crohns Colitis. 2020;14:1547–1557. doi: 10.1093/ecco-jcc/jjaa084. [DOI] [PubMed] [Google Scholar]
- Zhu C., Huang K., Bai Y., Feng X., Gong L., Wei C., Huang H., Zhang H. Dietary supplementation with berberine improves growth performance and modulates the composition and function of cecal microbiota in yellow-feathered broilers. Poult. Sci. 2021;100:1034–1048. doi: 10.1016/j.psj.2020.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.