Summary
Portal hypertension, defined as increased pressure in the portal vein, develops as a consequence of increased intrahepatic vascular resistance due to the dysregulation of liver sinusoidal endothelial cells (LSECs) and hepatic stellate cells (HSCs), frequently arising from chronic liver diseases. Extrahepatic haemodynamic changes contribute to the aggravation of portal hypertension. The pathogenic complexity of portal hypertension and the unsuccessful translation of preclinical studies have impeded the development of effective therapeutics for patients with cirrhosis, while counteracting hepatic and extrahepatic mechanisms also pose a major obstacle to effective treatment. In this review article, we will discuss the following topics: i) cellular and molecular mechanisms of portal hypertension, focusing on dysregulation of LSECs, HSCs and hepatic microvascular thrombosis, as well as changes in the extrahepatic vasculature, since these are the major contributors to portal hypertension; ii) translational/clinical advances in our knowledge of portal hypertension; and iii) future directions.
Keywords: HSC, LSEC, fibrosis, angiogenesis, liver stiffness, Rho-kinase, NO, FXR, VEGF, statins, NSBB, TIPS
Abbreviations: ACE2, angiogenesis-converting enzyme 2; ACLF, acute-on-chronic liver failure; AT1R, angiotensin II type I receptor; β1-AR, β1-adrenergic receptor; β2-AR, β2-adrenergic receptor; β-Arr2, β-arrestin 2; CCl4, carbon tetrachloride; CCL2, chemokine (C-C motif) ligand 2; CLD, chronic liver disease; CSPH, clinically significant portal hypertension; Dll4, delta like canonical Notch ligand 4; ECM, extracellular matrix; eNOS, endothelial nitric oxide synthase; EUS, endoscopic ultrasound; FXR, farnesoid X receptor; HCC, hepatocellular carcinoma; HRS, hepatorenal syndrome; HSCs, hepatic stellate cells; Hsp90, heat shock protein 90; HVPG, hepatic venous pressure gradient; JAK2, Janus kinase 2; KO, knockout; LSEC, liver sinusoidal endothelial cells; MLCP, myosin light-chain phosphatase; NET, neutrophil extracellular trap; NO, nitric oxide; NSBBs, non-selective beta blockers; PDE, phosphodiesterase; PDGF, platelet-derived growth factor; PKG, cGMP-dependent protein kinase; PIGF, placental growth factor; TIPS, transjugular intrahepatic portosystemic shunt; VCAM1, vascular cell adhesion molecule 1; VEGF, vascular endothelial growth factor
Key points.
-
•
Portal hypertension, the consequence of increased hepatic resistance and splanchnic hyperperfusion, is a driver of serious complications in cirrhosis.
-
•
The dysregulation of LSECs and HSCs is the main feature that leads to increased vascular resistance, but hepatic microvascular thrombosis also plays a significant role in the development of portal hypertension.
-
•
Splanchnic hyperperfusion is the result of severe changes in the extrahepatic vasculature (including neoangiogenesis) and hypocontractility of the splanchnic arteries.
-
•
The gut-liver-axis plays an important role in the development of complications of portal hypertension, although the mechanical increase of portal pressure does not directly impair the gut barrier long-term.
-
•
Diagnosis of clinically significant portal hypertension is possible using extracellular matrix-derived biomarkers, as well as liver and spleen stiffness measurements.
-
•
Novel endoscopic ultrasound-guided pressure measurement offers a good alternative, but hepatic venous pressure gradient measurement remains the gold standard.
-
•
Cell-specific drug-delivery and combinatorial therapies targeting the extrahepatic and intrahepatic vascular compartments are emerging as the best treatment strategies for portal hypertension.
Introduction
Portal hypertension is defined as increased pressure within the portal vein, which is the blood vessel connecting the outflow of the gastro-intestine and spleen (splanchnic organs) and the liver. Portal hypertension develops as a consequence of increased intrahepatic vascular resistance due to impaired hepatic sinusoidal circulation, most frequently1 arising from chronic liver diseases (CLDs). CLDs cause structural alterations of the liver via increased extracellular matrix (ECM) accumulation and turnover (fibrosis) and changes of the cellular phenotypes, associated with dysfunction of liver sinusoidal endothelial cells (LSECs), activated hepatic stellate cells (HSCs) and inflamed resident or infiltrating macrophages.2 These changes induce increased intrahepatic resistance, which increases the pressure in the portal vein (portal hypertension) and is the initial step towards complications of CLD. As a secondary event, portal hypertension induces splanchnic and systemic arterial vasodilation,3 leading to the development of a hyperdynamic circulatory syndrome and thereby aggravating and driving clinically detrimental complications.4 In recent years, the basic mechanisms of LSEC and HSC dysregulation have been extensively studied and potential therapeutic targets have been proposed. However, due to the complexity of the pathogenesis of portal hypertension and unsuccessful translation of preclinical studies to the human setting, development of effective therapeutics requires further mechanistic insight.
Portal hypertension is the driver of complications in cirrhosis, such as ascites and gastro-oesophageal varices (which can haemorrhage), as well as hepatic encephalopathy due to portosystemic shunting, hepatorenal syndrome and hypersplenism.5 Patients with complications of portal hypertension show repeating readmissions in the hospital and are described as having unstable decompensated cirrhosis. Another area of active investigation in recent years is the mechanism of systemic inflammation, which is a cause and consequence of acute decompensation in cirrhosis, and closely associated with pre-acute-on-chronic liver failure (ACLF). Ultimately, ACLF develops with a dramatically high mortality rate of approximately 40% at 28 days.6
In this review article, we will discuss: i) cellular and molecular mechanisms of portal hypertension, focusing on dysregulation of LSECs, HSCs and hepatic microvascular thrombosis, as well as the changes in the extrahepatic vasculature; ii) translational/clinical advances in our knowledge of portal hypertension, and iii) future directions.
Cellular & molecular mechanisms of portal hypertension
Hepatic cells
Among the hepatic cells involved in the development of portal hypertension, LSECs and HSCs are directly involved in the increased hepatic resistance. Thus, their modulation may both relieve the pressure and in the longer run ameliorate fibrosis.
LSEC dysfunction
Normal LSEC function is necessary for liver homeostasis (Fig. 1). This section discusses factors contributing to LSEC dysfunction and characteristics of dysfunctional LSECs, as well as the mechanisms that lead to LSEC dysfunction.
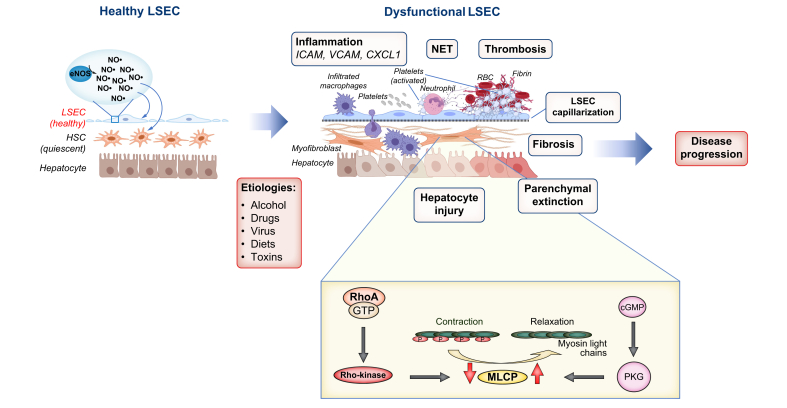
Fig. 1.
Liver sinusoidal cell dysfunction in liver pathogenesis.
Normal LSEC function is necessary for liver homeostasis. Various aetiologies can cause LSECs to become dysfunctional, leading to disease progression. Capillarisation is the loss of fenestrae and appearance of a basement membrane in LSECs. eNOS-derived NO plays a pivotal role in liver homeostasis by regulating vascular tone, maintaining fenestrae, maintaining HSCs in a quiescent state and blocking platelet attachments to endothelial cells among other functions. LSEC dysfunction often precedes pathological events, including inflammation, NET formation, microvascular thrombosis, parenchymal extinction (regions of tissue loss and fibrosis secondary to vascular obstruction and microvascular thrombosis), hepatocyte injury and fibrosis, leading to the development of portal hypertension. By contrast, in HSCs, calcium-independent contraction is mainly regulated by MLCP, which is inhibited by the RhoA/Rho-kinase pathway and activated by the NO/PKG-pathway. CXCL1, chemokine (C-X-C motif) ligand 1; HSCs, hepatic stellate cells; ICAM, intercellular adhesion molecule; LSECs, liver sinusoidal endothelial cells; MLCP, myosin light-chain phosphatase; NO, nitric oxide; NET, neutrophil extracellular trap; PKG, cGMP-dependent kinase; VCAM, vascular cell adhesion molecule.
Capillarisation: LSECs have fenestrae of approximately 0.1 microns organised into groups of sieve plates, which facilitate the transport of macromolecules from the hepatic sinusoids to the space of Disse, then to HSCs and hepatocytes. Additionally, a unique feature of LSECs that distinguishes them from endothelial cells in other organs is the lack of a basement membrane, which allows efficient movement of macromolecules between the lumen of the sinusoid and the space of Disse.7 Loss of fenestrae and the appearance of a basement membrane in LSECs is termed “capillarisation”.8,9 Vascular endothelial growth factor (VEGF) is known to be a key factor that maintains the fenestrae in the absence of a basement membrane10 through endothelial nitric oxide synthase (eNOS)-derived nitric oxide (NO) signalling.11 A study showed that removal of VEGF signalling in transgenic mice, in which liver-specific secretion of a soluble VEGF decoy receptor inactivates endogenous VEGF, results in a loss of LSEC fenestration and portal hypertension, as well as HSC activation independent of hepatic parenchymal damage. Administration of VEGF to these mice ameliorated portal hypertension.12 In addition, the composition of collagen in the space of Disse may play a role in the maintenance or loss of endothelial fenestration.13 A recent study in mice showed that delta like canonical Notch ligand 4 (Dll4), a ligand of the Notch signalling pathway that is predominantly expressed in endothelial cells, promotes LSEC capillarisation by basement membrane formation.14 Additionally, this study showed that DLL4 knockdown in a human LSEC cell line decreased ECM expression while its overexpression led to an increase in ECM proteins including collagens, fibronectin and laminin, confirming the role of DLL4 in LSEC capillarisation.14
Regulation of NO production: The ability of LSECs to generate NO is one of the most recognised indicators of LSEC function, given a wide spectrum of pivotal homeostatic functions mediated by eNOS-derived NO, including control of hepatic vascular tone, inhibition of thrombosis, and blocking HSC activation during fibrogenesis15 (Fig. 1). In liver diseases, it is well known that eNOS-derived NO production and/or NO bioavailability are significantly reduced, contributing to liver disease pathogenesis. A recent study identified a novel mechanism of eNOS regulation by β-arrestin2 (β-Arr2), demonstrating that decreased β-Arr2 levels in cholestatic LSECs contribute to reduced eNOS activity and sinusoidal portal hypertension in mice,16 while overall expression of β-Arr2 (at protein and mRNA levels) increases in human and experimental cirrhosis.17 Overexpression of β-Arr2 in LSECs significantly increases NO production, suggesting a role of β-Arr2 in eNOS activation and NO production.
Regulation of eNOS activity can be aetiology specific, given that eNOS is regulated by interactions with several proteins, which may themselves be regulated uniquely in different disease conditions. Heat shock protein 90 (Hsp90) activates eNOS, leading to NO production.18 Recently, we reported a mechanism of alcohol-driven LSEC dysfunction.19 In this study, we showed that LSECs can metabolise ethanol and that ethanol can increase Hsp90 acetylation through enhanced production of acetyl-CoA, a substrate for protein acetylation, by the action of alcohol dehydrogenase 1 (ADH1) and cytochrome P450 2E1 (CYP2E1).19 Further, acetylation of Hsp90 decreases its interaction with eNOS, decreasing production of eNOS-derived NO, while a de-acetylation mutant of Hsp90 increases an interaction with eNOS, leading to NO production. Overexpression of histone deacetylase 6 (HDAC6), an Hsp90-specific de-acetylase enzyme,20 in liver endothelial cells in mice increased the association of Hsp90 with eNOS and increased NO production, resulting in amelioration of alcohol-induced liver injury.19 These observations indicate that LSEC dysfunction can be treated in an aetiology-specific manner.
Autophagy: Autophagy is a highly conserved and controlled intracellular process involving the degradation and recycling of cellular components in lysosomes. A recent study by Ruart et al.21 showed that autophagy is important for LSEC homeostasis. Decreased autophagy in mice with endothelial cell-specific Atg7 deletion was associated with increased liver fibrosis and oxidative stress, as indicated by decreased expression of antioxidant enzymes such as superoxide dismutase, catalase and glutathione peroxidases, as well as those regulated by the nuclear factor-erythroid 2-related factor 2 (Nrf2). In addition, a phosphorylated (active) form of eNOS was decreased in endothelial-specific Atg7 knockout (KO) mice with acute liver injury induced by carbon tetrachloride (CCl4). These results indicate that autophagy in liver endothelial cells has a protective role against liver injury.
Similarly, in non-alcoholic steatohepatitis, autophagy in LSECs seems protective against liver fibrosis progression and inflammation.22 Endothelial-specific Atg5 KO mice with diminished autophagic response, showed an increase in endothelial inflammation (hepatic vascular cell adhesion molecule 1 [VCAM-1], chemokine (C-C motif) ligand 2 [CCL2], and CCL5 expression), liver injury (increased alanine aminotransferase), hepatic cell death (increased cleaved caspase-3), and liver fibrosis in response to a 16-week high-fat diet, compared to a chow control diet. Deficiency in autophagic responses also resulted in increased liver fibrosis in response to intraperitoneal injection of CCl4 for 6 weeks .
Enhancing autophagy in LSECs is protective against liver injury. A study in mice showed that statins help to maintain LSEC function in response to ischaemia reperfusion-induced liver injury by increasing autophagy and subsequent induction of Kruppel-like factor 2 (KLF2) activity, which is important for the maintenance of microvascular function.23 However, autophagy does not seem to be protective for all liver cell types, since autophagy is involved in HSC activation by providing an energy source in pathological conditions.24 Taken together, these studies indicate that autophagy in LSECs is protective against LSEC dysfunction, liver injury, and fibrosis.
Aging: Aging is a critical biological factor that influences LSEC function. With increases in age, there are major morphological changes in LSECs, including increased LSEC thickness and reduced fenestration, known as pseudocapillarisation.[25], [26], [27], [28] These age-related changes increase susceptibility to chronic liver injury29 and diabetes.30 In addition, aging increases the occurrence of portal hypertension and liver fibrosis in rats, as demonstrated by comparisons of aged rats (16 months) to young rats (1 month).29
Given these insights, age-related pseudocapillarisation could represent a therapeutic target. A study by Hunt et al.30 assessed the porosity in cultured LSECs in response to multiple drugs that act on the pathways that influence NO, and showed that treatments with nicotinamide mononucleotide, sildenafil, and 7-ketocholesterol increased fenestration porosity and frequency in LSECs isolated from young and old mice. Such drugs could potentially be used for the treatment of age-associated susceptibility to liver fibrosis and portal hypertension.
Hepatic stellate cells
HSCs are another unique cell type in the liver. In the quiescent state these cells store vitamin A, but upon injury they are activated and transdifferentiate into a myofibroblastic phenotype.31 A seminal study by the group of Robert Schwabe demonstrated that 98% of myofibroblasts populating the fibrotic septa were derived from HSCs.32 There is a large amount of literature on HSCs (>4,800 citations in PubMed 2005-2020), as well as very well written reviews on how these cells are activated and their role in the development of liver fibrosis.31,33 However, their role in portal hypertension has been investigated less extensively (>200 citations in PubMed 2005-2020), though fibrosis is associated with liver stiffness, angiogenesis and contraction, which all link HSCs to portal hypertension.
Stiffness: Liver stiffness may induce a mechanical increase in hepatic resistance and at least aggravate portal hypertension. The physical and chemical properties of the environment, in which HSCs are embedded, are also important for their activation,34 at least partly via Src and RhoA pathways.35 The majority of the fibrotic material is synthesised by HSCs, and together with other cells they contribute to the perpetuated remodelling of the matrix, reviewed elsewhere.36,37 The stiffness of the matrix is provided, among other mechanisms, by the crosslinking of collagens, which leads to a less reversible fibrosis.38 The stiffness itself may further boost the activation of HSCs, and the activated HSCs may migrate to those fibrosis spots due to so-called durotaxis,39 which has been shown for fibroblasts,40 and is likely to occur in HSCs as well. Moreover, the swelling of HSCs, e.g. in the presence of hyperammonia, may activate them and induce their contraction.41 This may further lead to aggravation of fibrosis and thereby increase liver stiffness, one of the main features of clinically significant portal hypertension (CSPH) in humans.42
Angiogenesis: HSCs are the hepatic pericytes involved in vessel formation and stabilisation.43 In this role, HSCs follow the transformation of LSECs upon injury (see above) and also drive LSEC transformation by releasing VEGF, which works in a paracrine and autocrine manner on LSECs and HSCs.43 Especially, platelet-derived growth factor (PDGF) is an important factor, which not only recruits HSCs but also boosts their fibrogenic potential. The boost in hepatic angiogenesis is probably meant to be a repair mechanism, but it aggravates the liver pathology and does not alleviate portal hypertension.43,44 The reason is that these newly formed vessels are different from the sinusoidal vasculature, and do not increase the blood perfusion of the cirrhotic liver, but they further impair the homeostasis of hepatocytes and aggravate liver damage, further fuelling fibrosis and inflammation.45 In experimental work, several anti-angiogenic strategies have been shown to be beneficial for portal hypertension, which could be partly attributed to improved fibrosis.[46], [47], [48], [49] Further targets to be addressed may be Vasohibin-150 and placental growth factor (PlGF).51 But in these studies, their effect on portal hypertension was at least partially due to their extrahepatic anti-angiogenic effect. Since hepatic resistance is at least partly dependent on fibrosis, it is difficult, or even impossible to separate the role of intrahepatic angiogenesis on portal hypertension from its role on fibrosis. Along with the transdifferentiation of HSCs, the fibrogenic and angiogenic potential increases with the development of a myofibroblastic apparatus enabling contraction. Contraction is the dynamic part of hepatic resistance.
Contraction: Contraction of HSCs narrows the sinusoids and thereby further increases hepatic resistance, causing and aggravating portal hypertension. Activated HSCs may show increased contraction stimulated by different vasoconstrictors. The contraction of myofibroblasts in general may occur in 2 ways (Fig. 1). One is the short-term, strong and calcium-dependent contraction, which is induced by myosin light-chain kinase (MLCK), which is usually mediated by G-protein coupled receptors.52 In particular, endothelin and catecholamines are known to induce such contractions. But myofibroblastic cells can also control vascular tone, which requires a steady and slow mechanism that is mainly regulated by myosin light-chain phosphatase (MLCP). The regulation of MLCP is crucial for determining vascular tone and thereby hepatic resistance.52
The activity of MLCP is inhibited by Rho-kinase, and thereby the contractile tone is increased.52 Rho-kinase seems to be crucial for HSC function, not only in contraction but also in fibrosis, as shown by the effect of its direct inhibition.53,54 Moreover, this master regulator of vascular function is so far downstream that many receptors and their downstream pathways regulate its function.55 Rho-kinase activity is regulated by many receptors, such as angiotensin II type I receptor (AT1R),56 β-adrenergic receptor (β-AR),57 urotensin-receptors,58 sphingosine-1-phosphate receptor 2 (S1P-R2),59,60 neuropeptide Y receptor (NPYR),61 and Mas-receptor,62,63 among others. But the intracellular pathways are also important and may reveal potential therapeutic targets, such as RhoA-activation or Gs/Adenylyl-cyclase.
On the other side, MLCP activity is enhanced by cGMP-dependent kinase (PKG), which is induced by cGMP.64 The main pathway of PKG activation is by the diffusion of NO supplied by LSECs.64 But cGMP can be regulated even in HSCs by different mechanisms. Phosphodiesterase (PDE), especially PDE5, can degrade cGMP, while soluble guanylate cyclase can increase its concentration. Subsequently, changes in cGMP level affect PKG activity, HSC contractility and thereby modulate hepatic resistance.[65], [66], [67], [68]
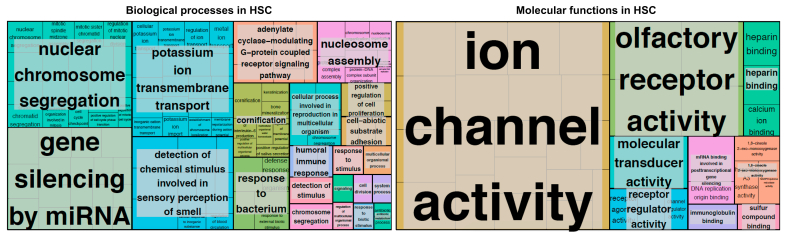
These processes represent the current understanding of the role of HSCs in the development of portal hypertension, but this may be biased by current knowledge and technology, with HSCs being far more complex. One example is the role of Rev-erb-alpha which is a gene regulator of the circadian rhythm. Indeed, Rev-erb-alpha also determines the phenotype and behaviour of HSCs. However, a direct manipulation of this mechanism also decreases portal hypertension in vivo.69 To illustrate the complexity, Fig. 2 shows the predicted processes (Fig. 2A, Table S1) and functions (Fig. 2B, Table S2) of HSCs, based on gene expression analysed by RNA-sequencing of murine-activated HSCs. These plots highlight the representative magnitude of specific processes and functions according to the number of genes expressed and underline the necessity of further research in this specific and clinically relevant field.
Fig. 2.
Biological processes and molecular functions in primary human HSCs.
Treemap representation of biological processes (A) and molecular functions (B) revealed by RNA-sequencing (transcriptome analysis) of human primary HSCs. The Treemap plot shows a two-level hierarchy of GO terms. Each rectangle is a GO term cluster representative. The representatives are joined together to loosely related GO terms and visualised. The size of the rectangles reflects the significance (p value) of the respective GO term. GoRilla207 was used in combination with REVIGO208 and R-studio (V1.3.1093) with packages TmPlot and TreeMap (R-Studio Team (2020). http://www.rstudio.com/) for the visualisation and GO term determination. The details of the pathways involved in the biological processes are found in Table S1 and the molecular functions in Table S2. GO, gene ontology; HSCs, hepatic stellate cells.
Hepatic microvascular thrombosis
A growing body of recent studies has suggested that thrombosis is one of the key pathological factors mediating portal hypertension. Previously, cirrhosis was thought to be an inherently anti-coagulated state because of decreased coagulation factor production by damaged hepatocytes and decreased platelet counts (i.e. thrombocytopenia). However, an accumulating body of evidence indicates that cirrhosis represents a re-balanced or even a procoagulant state.
Hepatic vascular thrombosis
What is the consequence of thrombi formation in the liver? A growing number of studies suggest the importance of intrahepatic microvascular thrombosis for fibrosis progression and portal hypertension.70 This observation connecting thrombosis and liver fibrosis was first described as “parenchymal extinction” in pathological specimens of human liver cirrhosis.71 Subsequently, a study in mice with CCl4-induced liver injury suggested that blood clotting is involved in the fibrotic response of the liver, given the observations of sinusoidal deposition of fibrin/fibrinogen and fibronectin in the damaged liver in the short-term, and deposition in fibrous septa during long-term liver damage.72 The critical role of blood clotting in the process of fibrogenesis was also shown in a study with rats, in which administration of a thrombin antagonist (SR182289) significantly decreased CCl4-induced liver fibrosis.73 Additionally, mice deficient in the prothrombinase fgl2/fibroleukin, an enzyme responsible for the conversion of prothrombin to thrombin, exhibited decreased fibrin deposition and necrosis in a model of viral hepatitis, again linking thrombosis to liver fibrogenesis.74 Further, treatment with anticoagulant drugs such as nadroparin and enoxaparin (low-molecular-weight heparins) led to a decrease in liver fibrosis in rats after bile duct ligation,75,76 thioacetamide administration (in which aspirin was also shown to reduce fibrosis), and CCl4-administration (in which another low-molecular-weight heparin, dalteparin, was shown to decrease fibrosis).77 A mechanistic study in rat models of liver injury/fibrosis with CCl4 or thioacetamide, with and without enoxaparin, demonstrated reduced portal pressure, reduced HSC activation, reduced fibrosis, and reduced fibrin deposition in enoxaparin-treated rats compared to control rats, possibly through a reduction of thrombosis.78 However, enoxaparin may not be effective in the late stages of cirrhosis, as suggested by a study showing no amelioration or improvement in liver fibrosis, liver function, and portal pressure in cirrhotic rats.79 Rivaroxaban, an anticoagulant drug that inhibits an active form of factor X, thereby inhibiting the generation of thrombin, has also been shown to reduce portal pressure in cirrhotic rats (induced by thioacetamide and CCl4), likely by reducing HSC activation, endothelial dysfunction, and microvascular thrombosis rather than via a direct effect on fibrosis.80 Collectively, anticoagulation therapy seems beneficial for the treatment of liver fibrosis and portal hypertension, although it is dependent on the stage of cirrhosis.
Congestive hepatopathy
Chronic hepatic congestion, or congestive hepatopathy, is a cause of portal hypertension that occurs in conditions that disturb efficient blood flow in the liver, such as congestive heart failure and Budd-Chiari syndrome. Mice with a partial inferior vena cava ligation develop post-hepatic portal hypertension and can be used as a model to mimic congestive hepatopathy. Using this model, Simonetto et al. demonstrated that chronic congestion induces liver fibrosis through sinusoidal thrombosis and mechanical forces.81 Pharmacologic treatment with warfarin, an anticoagulant agent that blocks vitamin K epoxide reductase, as well as genetic inhibition of the clotting cascade (transgenic mice overexpressing tissue factor pathway inhibitor [SM22a-TFPI]), decreased hepatic thrombosis and liver fibrosis. Mechanistically, the authors showed that increased fibrin deposition together with sinusoidal mechanical stretch of HSCs facilitates fibrin-fibronectin complex formation at HSCs, leading to ECM deposition. Although warfarin treatment can decrease fibrosis, it did not significantly reduce portal hypertension in congestive hepatopathy. Chronic hepatic congestion also mechanically stimulates LSECs to release angiocrine factors, such as the chemokine (C-X-C motif) ligand 1 (CXCL1) and the neutrophil chemotactic chemokines; it also facilitates neutrophil recruitment at the site of the thrombus, leading to the formation of neutrophil extracellular traps (NETs). Inhibition of NET formation by knocking out neutrophil elastase, which is essential for NET formation, significantly decreased liver fibrosis and portal hypertension in mice, again supporting the role of hepatic thrombosis in liver fibrosis and portal hypertension.82
Extrahepatic circulation
In portal hypertension, increased portal blood inflow from the splanchnic circulation augments portal pressure and thereby contributes to the maintenance and exacerbation of portal hypertension.83 Portosystemic collaterals contribute to the development of varices and the delivery of noxious substances from the portal circulation directly to the cerebral vasculature without hepatic detoxification, thus contributing to hepatic encephalopathy.84 These changes together with the arterial vasodilation in the splanchnic circulation play a critical role in increasing blood flow to the portal vein.
Pathological angiogenesis
It was thought that portosystemic collaterals are an opening of pre-existing vessels, but a study by Sieber and colleagues was the first to indicate the evidence of angiogenesis-driven collateral vessel formation in portal hypertensive rats using a device consisting of collagen type I-filled teflon ring, which was implanted into the mesenteric bed for the assessment of angiogenesis.85 Although initial collateral formation likely develops due to the opening of pre-existing vessels, accumulating evidence has resulted in a consensus that most portosystemic collateral vessels are formed through angiogenesis.
It is thought that these portosystemic collateral vessels are “pathological” because they further increase portal vein inflow and increase the likelihood and the incidence of variceal haemorrhage.43,70,86 Thus, numerous studies have attempted to target pathological angiogenesis in experimental models of portal hypertension and cirrhosis through a variety of approaches, such as targeting VEGF (with anti-VEGFR2 or a combination of anti-VEGF [rapamycin]/anti-PDGF [imatinib]), PlGF,51 apelin,87 cannabinoid,88 peroxisome proliferator-activated receptor (PPAR)α and hedgehog signalling, or using sorafenib.89,90 However, the reduction of collateral vessels does not always result in a decrease in portal pressure to the normal level, since this reduction does not significantly change the portal blood flow.51,91 In addition, clinical studies of an anti-angiogenic approach such as anti-VEGF treatment have given unsatisfactory results because this approach also blocks the homeostatic activity of VEGF, such as wound healing angiogenesis, and other non-specific effects of VEGF that are important for vascular homeostasis. Thus, targeting only “pathological angiogenic activity” may be a novel approach to ameliorate portal hypertension.
A recent study by De Gottardi and colleagues92 showed that Paneth cells in the lining of the small intestine regulate mesenteric angiogenesis and portal hypertension. Paneth cells secrete antimicrobial peptides that mediate host-microbe interactions, keeping a balance between intestinal microflora and enteric pathogens. Using a mouse model of conditional ablation of Paneth cells (Math1Lox/LoxVilcreERT2 mice with tamoxifen), it was shown that Paneth cell-ablation resulted in a significant reduction in portosystemic collateral vessels, portal pressure, and CD31-positive vessels in mice following partial portal vein ligation. Thus, in response to intestinal flora and microbiota-driven factors, Paneth cells secrete not only antimicrobial peptides, but also pro-angiogenic signalling molecules, promoting intestinal and mesenteric angiogenesis and regulating portal hypertension.
Extrahepatic vasodilation
Besides the pathological angiogenesis and impaired gut-vascular barrier, the tone of arterial vessels preceding the portal circulation is decreased.3 The changes occurring in the contractile pathway oppose those in HSCs, which renders the management of portal hypertension very difficult.1,3 In particular, the circulating and local levels of NO are increased in these vessels,93 which – among other mechanisms – are related to shear stress in the endothelium secondary to portal congestion and bacterial translocation.[94], [95], [96], [97] Recent data demonstrating increased levels of NO in the portal vein and its strong correlation with lipopolysaccharide in human cirrhosis may underline this hypothesis,97 along with experimental evidence published by different groups.[97], [98], [99]
Indeed, in parallel to the increased NO synthesis and bioavailability in these vessels, the vasocontractile pathways are also impaired.3 It has been demonstrated that the RhoA/Rho-kinase pathway is defective and less active.100 This is at least partly due to changes in the post-receptor regulation of vasoconstrictor receptors, such as AT1R, which is desensitised by increased β-Arr2 binding.56 Moreover, in the renin-angiotensin-system there is a shift towards the activated angiotensin-converting enzyme 2 (ACE2)-/masR axis in the splanchnic vessels in parallel to the desensitised AT1R.62,101 It could be shown that neuropeptide Y, as a co-neurotransmitter of catecholamines and angiotensin-2, could cause vascular contraction.61 These data suggest that this vascular dysfunction is not structural and is to a large extent reversible.61 Similarly, the shift towards the ACE2-/masR axis was reduced in patients after liver transplantation, demonstrating that the diseased liver plays a causative role in these changes.101
Taking into the account the contrary regulation of the extrahepatic and intrahepatic mechanisms, cell-specific and hepatic delivery of vasodilators is extremely important. There have been attempts at targeting Rho-kinase in HSCs, which do not elicit severe systemic complications.102,103 Yet these are still experimental.
Gut-liver axis and portal hypertension
The gut-liver axis has been increasingly investigated in recent years, partly thanks to technological advances in the methods used to investigate the microbiota.104 Hence, we performed an analysis of the published literature on the gut-liver axis and portal hypertension from the last 15 years and plotted the key words (after removing the random words) in order to highlight the main findings or opinions (Fig. 3). Besides the general mechanisms of bacterial intestinal translocation, a wide spectrum of words appeared, such as inflammation, endotoxemia, hepatic encephalopathy, small bowel dysbiosis, increased permeability, mesenteric neovascularization, bacterial overgrowth, peritonitis, non-selective beta blockers, Apelin and so on (Fig. 3). Interestingly, NAFLD is mentioned in a large number of patients, although alcohol-related cirrhosis is much more closely linked to the gut-liver-axis. This calls for the clear prioritisation of this aetiology in future research.
Fig. 3.
Word cloud representing the most frequent keywords of abstracts with the keyword gut-liver axis.
The size of the words within the word cloud correlates with the frequency of the respective word in the analysed abstract text.
Gut barrier function in portal hypertension
As outlined above, gut barrier function is impaired in the presence of portal hypertension in cirrhosis. There are several reasons for this. First the aetiology of cirrhosis (alcohol, diet, decreased bile flux) or of the non-cirrhotic portal hypertension (splanchnic or hepatic inflammatory processes) may disrupt the gut epithelium directly or via increased inflammation.[105], [106], [107] The venous congestion associated with splanchnic vasodilation and splanchnic neoangiogenesis may impair the gut-vascular barrier and increase permeability in the gut. The complications of portal hypertension in cirrhosis, e.g. renal and immune cell dysfunction, may also facilitate bacterial translocation.107 It is widely accepted that bacterial translocation exists and is of major relevance in cirrhosis.107 Yet it is important to emphasise that a mechanical increase in portal pressure, in the absence of inflammatory processes or cirrhosis, is not associated with increased disruption in the gut barrier.108
Secondary to this disruption of the gut barrier, the translocation of bacterial components or bacteria into the circulation continuously activate the immune system. This may lead to chronic systemic inflammation, which may take place at multiple levels: in the portal circulation, predisposing to splanchnic thrombosis; in the liver, promoting inflammation and fibrosis; and in other organs, inducing organ dysfunction.97,[109], [110], [111] Translocation into the lymphatic system is also important, as it may be associated with spontaneous bacterial peritonitis and other spontaneous infections.112,113
The gut barrier impairment is, in addition to the aforementioned pathophysiological mechanisms, related to changes in the gut microbiota itself.
Effects of microbiota on the liver
The gut microbiota harbours the largest pool of genetic material in the human body with more than 1013 microbes with an extremely high metabolic activity.105,114 Nowadays, there is no doubt that changes in the microbiota influence cirrhosis (summarised in several recent reviews) and that cirrhosis induces profound changes in the microbiota.107,115,116 However, the role of portal hypertension in this setting is not yet well understood. Indeed, it is difficult to clearly attribute specific changes in microbiota to portal hypertension. By contrast, there is evidence showing that the microbiota and the bile acid pool, which can also target the farnesoid X receptor (FXR) on liver cells, may aggravate or alleviate portal hypertension and its complications.110,[117], [118], [119], [120], [121] Secreted bile acids are modified by the intestinal microbiota leading to decreased FXR stimulation which may increase the contractility of HSCs and thereby portal hypertension.117,119 This is evident when FXR agonists are administered.110,[117], [118], [119], [120], [121] In addition, the microbiome is present in other compartments besides the gut in the absence of infection. In recent studies, blood, liver, bile and ascitic fluid have been analysed in patients with cirrhosis.122 Indeed, in patients with complications of portal hypertension such as ascites, the composition of the circulating microbiome in the portal vein compared to the hepatic vein and the systemic circulation varies, while the DNA of specific species was correlated with inflammatory markers, which was not the case for ascites.123,124 In ascites, only the evident infection or bacterascites, but not bacterial DNA, seems to be relevant for outcome.124 But in the blood circulation, the specific microbial members may aggravate hepatic inflammation,123 and thereby probably hepatic resistance. This is supported by patient data showing that liver stiffness (a surrogate marker of portal hypertension and inflammation) and levels of inflammatory markers were higher in the hepatic vein than in the portal vein. These patients more often developed organ failure and had worse outcomes, even after an adequate decrease of portal pressure using transjugular intrahepatic portosystemic shunt (TIPS).125 The existing evidence linking the gut microbiota and microbiome to portal hypertension is still scarce and substantial work is required to catch up with the available technological advances.
Translational/clinical advances in our knowledge of portal hypertension
Portal hypertension represents a hallmark in the course of chronic liver disease, as the aforementioned changes and mechanisms lead to the perpetuation of injury, guaranteeing the progression and worsening of prognosis in cirrhosis. For this reason, for more than a century, cirrhosis was believed to be irreversible. Yet, advances in treatment, particularly for viral hepatitis, have shown that cirrhosis and portal hypertension may be reversible if the aetiology of CLD is treated. This has been shown for the treatment of autoimmune hepatitis, HBV and HCV, and alongside weight loss,[126], [127], [128] and abstinence. Yet, the development of portal hypertension above a certain threshold is a predictor of complications, especially in the presence of risk factors (e.g. obesity, alcohol intake, etc.). Indeed, recently it was shown in a large prospective series of patients that severe portal hypertension induces an unstable course of disease after an acute decompensating event,129 at least partly due to bacterial infections,6 and secondary to variceal bleeding episodes in at least 20% of cases.130
A pressure gradient of more than 10 mmHg between the portal and hepatic vein is characterised as CSPH, which marks the point at which portal hypertension is likely to cause clinical complications.131 Therefore, it is extremely important to diagnose CSPH and to monitor it upon treatment.
Diagnosis of clinically significant portal hypertension
Invasive tools
The gold standard for the diagnosis of CSPH is the invasive (using venous access) measurement of the gradient between the free and so-called wedged hepatic vein – associated practical issues are discussed elsewhere.131 Briefly, the wedge-pressure represents the conducted pressure of the sinusoids and therefore the portal pressure. Using this approach to measure the pressure gradient has advantages as it allows for a thorough assessment of systemic haemodynamics (Swan-Ganz) and even for a liver biopsy (if indicated).
Yet, the most reliable measurement is obtained by puncturing the portal vein using the transjugular-transhepatic approach.132 This approach is often used in the TIPS setting, but very rarely for diagnostic purposes. One potential reason to use a direct puncture is if the measurement of hepatic venous pressure gradient (HVPG) is not possible due to shunts and if it is not possible to reach a wedge-position.132
In recent reports the direct puncture of the portal vein using endoscopic ultrasound has been reported for sampling and a direct measurement of portal pressure, which seems to be safe and technically feasible.[133], [134], [135] This technique could aid patient management, especially when varices are present at endoscopy, although more research is needed.
Despite improvements, invasive tools are associated with higher costs and possible morbidity, which are particular drawbacks of liver biopsy. Therefore, significant efforts have been made to develop non-invasive tools.
Non-invasive tools
Besides HVPG as the gold standard, liver stiffness measurement using different elastographic techniques seems to play a central role in the diagnosis.136 Owing to technical advances, elastography can now be performed routinely using most ultrasound machines. The best validated for portal hypertension is Fibroscan, which is recommended by Baveno to guide screening endoscopy for varices, followed by 2D-Shear-wave elastography of Aixplorer, the last being superior in patients with ascites.42,[137], [138], [139] The combination of liver and spleen stiffness measurement seems to be the most accurate to assess CSPH and the effect of portal pressure-lowering strategies.42,125,138 One reason is that liver stiffness also depends on other mechanisms, such as inflammation, cholestasis or right heart congestion, of which the latter 2 can be ruled out using an ultrasound-based elastographic measurement.
Since patients with cirrhosis undergo mandatory hepatocellular carcinoma screening, ultrasound-based elastography may be convenient as a one-stop-shop diagnostic. Yet in many patients, MRI is also performed. Besides MR-elastography, there are novel protocols, such as the measurement of extracellular volume or the liver inflammation and fibrosis score, that do not require a specific device to measure portal pressure.[140], [141], [142] The measurement of extracellular volume is mainly validated for fibrosis, but it also correlates with portal pressure as shown in animals.[141], [142], [143] Still, significant efforts are required to make it ready for widespread clinical use, but it may be interesting as a one-stop-shop diagnostic approach.
The easiest approach is to use soluble biomarkers. In particular, ECM markers may be useful for the detection of CSPH and complications,36,[144], [145], [146] while inflammatory and haemodynamic markers,[147], [148], [149], [150] or even circulating miRNA are rather unspecific.[151], [152], [153] Maybe in the near future, signature molecules based on omics-techniques may help to improve the diagnosis of CSPH.
Treatment strategies for clinically significant portal hypertension
Despite the large numbers of patients affected by portal hypertension, there are only few therapeutic options for them.154 Moreover, the treatment strategies for portal hypertension are stratified according to the complications of portal hypertension rather than the portal hypertension itself. There are basically 2 larger groups of complications directly linked to the magnitude of portal hypertension, varices and ascites development. The established strategies are tested in multiple studies with hard endpoints (mortality) and thereby are better investigated than most treatments in other indications.
Established strategies
The current treatment strategies for varices development and bleeding include non-selective beta blockers (NSBBs), which were introduced for this indication 40 years ago by the group of Didier Lebrec.155,156 The rationale for NSBB use is to decrease splanchnic venous inflow and cardiac output, by blocking the β1-adrenergic receptor (β1-AR), and to induce vasoconstriction in the splanchnic region by blocking β2-AR. In the last decade, the use of carvedilol, brought into the field by the group of Peter Hayes,157,158 seems to be more effective, leading to higher haemodynamic response rates159 than the classical NSBBs.159,160 NSBBs are indicated for primary prophylaxis (to prevent bleeding once varices are present) and for secondary prophylaxis (to prevent re-bleeding). Baveno conferences have established very useful guidelines for the use of NSBBs over the last 30 years. While NSBBs may not be useful in the prevention of varices development, if CSPH is absent, as shown by the seminal paper of R. Groszmann,161 they have important benefits in the prevention of the variety of consequences of portal hypertension in patients with CSPH.160 This study is complex and showed a positive effect of NSBBs, because it was not designed as a “one size fits all” strategy. The authors first tested the haemodynamic response of patients to propranolol and in case of non-response patients received carvedilol. This is very important since it demonstrates that the use of drugs should be very differentiated (almost individualised) in portal hypertension. Yet, the effect of NSBBs on portal pressure is limited to an approximately 15% decrease; thus, they may not be sufficient for the prevention of bleeding of very large varices. Therefore, the local treatment of varices, using band ligation, may be as effective as NSBBs for the prevention of first bleeding.4
In addition to the case of large varices, NSBBs may be deleterious in the presence of refractory ascites, especially in patients with severe arterial hypotension, active infection or acute kidney injury, and at least a dose reduction is recommended.137 Therefore, the window of opportunity to use NSBBs for the treatment of complications of portal hypertension may be rather narrow.162 Generally speaking, NSBBs are contraindicated for acute events since the required portal venous decompression needs to be achieved fast at a large magnitude. Such effects are elicited by terlipressin and shunting procedures.163 Therefore, both are recommended to achieve haemostasis in patients with variceal bleeding. Terlipressin or other vasoconstrictors such as sandostatin are used in the acute setting of variceal haemorrhage, but also in the treatment of hepato-renal syndrome (HRS).137,164 HRS is the maximal form of kidney dysfunction in cirrhosis and is basically a functional failure caused by intrarenal vasoconstriction, but also at least partly by the decreased effective arterial blood volume following long-standing portal hypertension and hyperdynamic circulation.165 In addition, infections occur frequently as a result of complications of portal hypertension, such as bleeding130 and HRS,164 and conversely may further aggravate portal hypertension.166
Shunting procedures, mainly TIPS insertion, are the most effective measure to decrease portal pressure.167 TIPS was introduced by the group of Martin Rössle. Besides the effective decompression of the portal vascular compartment,167 a TIPS increases the effective arterial blood volume and thereby improves renal function. Although this effect is not immediate, it has been clearly demonstrated to improve renal function, increase sodium excretion and ameliorate ascites.[168], [169], [170]
This is a very short list of therapeutic options for the large number of patients affected by portal hypertension.154 There are many explanations for the imbalance between clinical need and available therapies, including issues of a health-economic, social and political nature. The consequence is that strategies to improve patient care in portal hypertension are based mainly on repurposing existing drugs or exploration of orphan drugs.154
There have recently been many disappointing studies in humans, despite very promising experimental data. There may be several reasons for this, ranging from strong differences between animal models and human disease to non-stringent experimental and clinical study designs. These unfortunate stories have used up huge resources, and the community should learn from them, starting with the NCX-1000,171,172 anti-angiogenic drugs,43 rifaximin,173,174 caspase-inhibitors,[175], [176], [177], [178], [179] and several more cases that have not yet been fully published.
Single drug treatments
Statins are a success story in cirrhosis, with experimental and clinical evidence now more than 10 years old.[179], [180], [181], [182] However, statins are entering clinical practice slowly, partly because of potential side effects,183,184 which can be bypassed by compound modifications.185 FXR agonists are another drug class that has shown efficacy for experimental portal hypertension [117], [118], [119], [120],186,187), while genetic predisposition may further suggest a beneficial effect188 and the first unpublished clinical attempts have been positive (e.g. PESTO). Anticoagulants may indirectly decrease portal pressure – by resolving thrombosis, preventing re-thrombosis and thereby preventing complications of portal hypertension – and even improve survival.[78], [79], [80],189 This was recently confirmed in a large cohort of patients receiving anticoagulation for atrial fibrillation.190 Finally, serelaxin is another promising approach, for which results are pending.[191], [192], [193]
Further possible strategies, which are already in the clinics are peroxisome proliferator-activated receptor agonists (fenofibrate, aligletazar, pioglitazone), which seem to have an effect on the hepatic (alpha) or collateral (gamma) circulation.[194], [195], [196] Soluble guanylate cyclase stimulators may also find a way into clinical practice, yet further validation is required.66 Urotensin-receptor antagonists have a very interesting profile, since they decrease hepatic resistance and increase splanchnic resistance, probably due to the differential effects of the receptor depending on its location.58,197,198 Janus kinase 2 (JAK2)-inhibition is another strategy of potential interest, yet more investigations are needed, since it may aggravate steatosis if not targeted to HSCs.[199], [200], [201], [202], [203], [204]
Taking into the account the contrary regulation of the extrahepatic and intrahepatic circulation, cell-specific and hepatic delivery of vasodilators is extremely important. There have been attempts at targeting Rho-kinase in HSCs, which do not induce extrahepatic effects.102,103 Yet these are still experimental and need to be confirmed in the human setting.
Combinatorial therapies
A possible strategy to improve efficacy is to combine drugs. This has already been well-implemented for secondary prophylaxis of variceal bleeding, combining NSBBs with local treatment of varices.137 Even in patients receiving TIPS, older data from the Sauerbruch group show that NSBBs improve portal hypertension.205 β3-adrenoceptor agonists are very attractive, since β3-adrenoceptor expression is increased only in activated HSCs and β3-adrenoceptor agonists show additive effects in combination with NSBBs.57,206 The concept of combinatorial therapies has been investigated in several Horizon 2020 projects (funded by the European Commission) such as LIVERHOPE (simvastatin and rifaximin) and in Decision (combination will be identified using omics-approach).
Conclusion and future directions
The main reasons for the lack of treatment strategies in the field of portal hypertension may be the complexity of the pathophysiological processes and the counterplay of the intrahepatic and extrahepatic systems. Therefore, there is a clear role for microbiome-targeted and omics-guided diagnosis and therapy for portal hypertension. In the era of minimally invasive approaches, personalised medicine and artificial intelligence, we need to open the field to scientists and experts from a wide range of backgrounds and liaise with them to develop effective systems medicine and personalised approaches. The introduction of advanced technologies, such as endoscopic ultrasound and cell-specific therapies that use drug-carriers to target either LSECs or HSCs, will significantly improve patient care.
Financial support
NIH grants (R01DK117597, 1R01AA025342 and R56DK121511) to YI and Deutsche Forschungsgemeinschaft (SFB TRR57 to P18, CRC 1382 to A09), European Union's Horizon 2020 Research and Innovation Programme (Galaxy, No. 668031; MICROB-PREDICT, No. 825694; DECISION, No. 847949), Eurostars (DeFiber, E!12350), IK and Societal Challenges - Health, Demographic Change and Wellbeing (LIVERHOPE, No. 731875), and Cellex Foundation (PREDICT) to JT.
Authors’ contributions
YI and JT both wrote the manuscript.
Conflict of interest
Jonel Trebicka has received speaking and/or consulting fees from Gore, Bayer, Alexion, MSD, Gilead, Intercept, Norgine, Grifols, Versantis, and Martin Pharmaceutical.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2021.100316.
Supplementary data
The following is the supplementary data to this article:
References
- 1.Bosch J., Groszmann R.J., Shah V.H. Evolution in the understanding of the pathophysiological basis of portal hypertension: how changes in paradigm are leading to successful new treatments. J Hepatol. 2015;62:S121–S130. doi: 10.1016/j.jhep.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwakiri Y., Groszmann R.J. The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule. Hepatology. 2006;43:S121–S131. doi: 10.1002/hep.20993. [DOI] [PubMed] [Google Scholar]
- 3.Hennenberg M., Trebicka J., Sauerbruch T., Heller J. Mechanisms of extrahepatic vasodilation in portal hypertension. Gut. 2008;57:1300–1314. doi: 10.1136/gut.2007.144584. [DOI] [PubMed] [Google Scholar]
- 4.Sauerbruch T., Schierwagen R., Trebicka J. Managing portal hypertension in patients with liver cirrhosis. F1000Res. 2018;7 doi: 10.12688/f1000research.13943.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trebicka J., Reiberger T., Laleman W. Gut-liver Axis links portal hypertension to acute-on-chronic liver failure. Visc Med. 2018;34:270–275. doi: 10.1159/000490262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trebicka J., Fernandez J., Papp M., Caraceni P., Laleman W., Gambino C. PREDICT identifies precipitating events associated with clinical course of acutely decompensated cirrhosis. J Hepatol. 2020 accepted. [Google Scholar]
- 7.Wisse E. An electron microscopic study of the fenestrated endothelial lining of rat liver sinusoids. J Ultrastruct Res. 1970;31:125–150. doi: 10.1016/s0022-5320(70)90150-4. [DOI] [PubMed] [Google Scholar]
- 8.Bhunchet E., Fujieda K. Capillarization and venularization of hepatic sinusoids in porcine serum-induced rat liver fibrosis: a mechanism to maintain liver blood flow. Hepatology. 1993;18:1450–1458. [PubMed] [Google Scholar]
- 9.Horn T., Christoffersen P., Henriksen J.H. Alcoholic liver injury: defenestration in noncirrhotic livers--a scanning electron microscopic study. Hepatology. 1987;7:77–82. doi: 10.1002/hep.1840070117. [DOI] [PubMed] [Google Scholar]
- 10.Funyu J., Mochida S., Inao M., Matsui A., Fujiwara K. VEGF can act as vascular permeability factor in the hepatic sinusoids through upregulation of porosity of endothelial cells. Biochem Biophys Res Commun. 2001;280:481–485. doi: 10.1006/bbrc.2000.4148. [DOI] [PubMed] [Google Scholar]
- 11.DeLeve L.D., Wang X., Hu L., McCuskey M.K., McCuskey R.S. Rat liver sinusoidal endothelial cell phenotype is maintained by paracrine and autocrine regulation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G757–G763. doi: 10.1152/ajpgi.00017.2004. [DOI] [PubMed] [Google Scholar]
- 12.May D., Djonov V., Zamir G., Bala M., Safadi R., Sklair-Levy M. A transgenic model for conditional induction and rescue of portal hypertension reveals a role of VEGF-mediated regulation of sinusoidal fenestrations. PloS One. 2011;6 doi: 10.1371/journal.pone.0021478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGuire R.F., Bissell D.M., Boyles J., Roll F.J. Role of extracellular matrix in regulating fenestrations of sinusoidal endothelial cells isolated from normal rat liver. Hepatology. 1992;15:989–997. doi: 10.1002/hep.1840150603. [DOI] [PubMed] [Google Scholar]
- 14.Chen L., Gu T., Li B., Li F., Ma Z., Zhang Q. Delta-like ligand 4/DLL4 regulates the capillarization of liver sinusoidal endothelial cell and liver fibrogenesis. Biochim Biophys Acta Mol Cell Res. 2019;1866:1663–1675. doi: 10.1016/j.bbamcr.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Iwakiri Y., Kim M.Y. Nitric oxide in liver diseases. Trends Pharmacol Sci. 2015;36:524–536. doi: 10.1016/j.tips.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu S., Luttrell L.M., Premont R.T., Rockey D.C. beta-Arrestin2 is a critical component of the GPCR-eNOS signalosome. Proc Natl Acad Sci U S A. 2020;117:11483–11492. doi: 10.1073/pnas.1922608117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schierwagen R., Dietrich P., Klein S., Uschner F.E., Ortiz C., Tyc O. Beta-arrestin-2 is increased in chronic liver injury. Proc Natl Acad Sci U S A. 2020 [Google Scholar]
- 18.Garcia-Cardena G., Fan R., Shah V., Sorrentino R., Cirino G., Papapetropoulos A. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature. 1998;392:821–824. doi: 10.1038/33934. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y., Sangwung P., Kondo R., Jung Y., McConnell M.J., Jeong J. Alcohol-induced Hsp90 acetylation is a novel driver of liver sinusoidal endothelial dysfunction and alcohol-related liver disease. J Hepatol. 2021 doi: 10.1016/j.jhep.2021.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scroggins B.T., Robzyk K., Wang D., Marcu M.G., Tsutsumi S., Beebe K. An acetylation site in the middle domain of Hsp90 regulates chaperone function. Mol Cell. 2007;25:151–159. doi: 10.1016/j.molcel.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruart M., Chavarria L., Camprecios G., Suarez-Herrera N., Montironi C., Guixe-Muntet S. Impaired endothelial autophagy promotes liver fibrosis by aggravating the oxidative stress response during acute liver injury. J Hepatol. 2019;70:458–469. doi: 10.1016/j.jhep.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammoutene A., Biquard L., Lasselin J., Kheloufi M., Tanguy M., Vion A.C. A defect in endothelial autophagy occurs in patients with non-alcoholic steatohepatitis and promotes inflammation and fibrosis. J Hepatol. 2020;72:528–538. doi: 10.1016/j.jhep.2019.10.028. [DOI] [PubMed] [Google Scholar]
- 23.Guixe-Muntet S., de Mesquita F.C., Vila S., Hernandez-Gea V., Peralta C., Garcia-Pagan J.C. Cross-talk between autophagy and KLF2 determines endothelial cell phenotype and microvascular function in acute liver injury. J Hepatol. 2017;66:86–94. doi: 10.1016/j.jhep.2016.07.051. [DOI] [PubMed] [Google Scholar]
- 24.Weiskirchen R., Tacke F. Relevance of autophagy in parenchymal and non-parenchymal liver cells for health and disease. Cells. 2019;8 doi: 10.3390/cells8010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DG L.E.C., Cogger V.C., McCuskey R.S., R D.E.C., Smedsrod B., Sorensen K.K. Age-related changes in the liver sinusoidal endothelium: a mechanism for dyslipidemia. Ann N Y Acad Sci. 2007;1114:79–87. doi: 10.1196/annals.1396.003. [DOI] [PubMed] [Google Scholar]
- 26.Le Couteur D.G., Cogger V.C., Markus A.M., Harvey P.J., Yin Z.L., Ansselin A.D. Pseudocapillarization and associated energy limitation in the aged rat liver. Hepatology. 2001;33:537–543. doi: 10.1053/jhep.2001.22754. [DOI] [PubMed] [Google Scholar]
- 27.Le Couteur D.G., Warren A., Cogger V.C., Smedsrod B., Sorensen K.K., De Cabo R. Old age and the hepatic sinusoid. Anat Rec (Hoboken) 2008;291:672–683. doi: 10.1002/ar.20661. [DOI] [PubMed] [Google Scholar]
- 28.Wake K., Sato T. "The sinusoid" in the liver: lessons learned from the original definition by Charles Sedgwick Minot (1900) Anat Rec (Hoboken) 2015;298:2071–2080. doi: 10.1002/ar.23263. [DOI] [PubMed] [Google Scholar]
- 29.Maeso-Diaz R., Ortega-Ribera M., Lafoz E., Lozano J.J., Baiges A., Frances R. Aging influences hepatic microvascular biology and liver fibrosis in advanced chronic liver disease. Aging Dis. 2019;10:684–698. doi: 10.14336/AD.2019.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunt N.J., Lockwood G.P., Warren A., Mao H., McCourt P.A.G., Le Couteur D.G. Manipulating fenestrations in young and old liver sinusoidal endothelial cells. Am J Physiol Gastrointest Liver Physiol. 2019;316:G144–G154. doi: 10.1152/ajpgi.00179.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsuchida T., Friedman S.L. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397–411. doi: 10.1038/nrgastro.2017.38. [DOI] [PubMed] [Google Scholar]
- 32.Mederacke I., Hsu C.C., Troeger J.S., Huebener P., Mu X., Dapito D.H. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kisseleva T., Brenner D.A. Anti-fibrogenic strategies and the regression of fibrosis. Best Pract Res Clin Gastroenterol. 2011;25:305–317. doi: 10.1016/j.bpg.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olsen A.L., Bloomer S.A., Chan E.P., Gaca M.D., Georges P.C., Sackey B. Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am J Physiol Gastrointest Liver Physiol. 2011;301:G110–G118. doi: 10.1152/ajpgi.00412.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gortzen J., Schierwagen R., Bierwolf J., Klein S., Uschner F.E., van der Ven P.F. Interplay of matrix stiffness and c-SRC in hepatic fibrosis. Front Physiol. 2015;6:359. doi: 10.3389/fphys.2015.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karsdal M.A., Manon-Jensen T., Genovese F., Kristensen J.H., Nielsen M.J., Sand J.M. Novel insights into the function and dynamics of extracellular matrix in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2015;308:G807–G830. doi: 10.1152/ajpgi.00447.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villesen I.F., Daniels S.J., Leeming D.J., Karsdal M.A., Nielsen M.J. Review article: the signalling and functional role of the extracellular matrix in the development of liver fibrosis. Aliment Pharmacol Ther. 2020;52:85–97. doi: 10.1111/apt.15773. [DOI] [PubMed] [Google Scholar]
- 38.Liu S.B., Ikenaga N., Peng Z.W., Sverdlov D.Y., Greenstein A., Smith V. Lysyl oxidase activity contributes to collagen stabilization during liver fibrosis progression and limits spontaneous fibrosis reversal in mice. FASEB J. 2016;30:1599–1609. doi: 10.1096/fj.14-268425. [DOI] [PubMed] [Google Scholar]
- 39.Sunyer R., Trepat X. Durotaxis. Curr Biol. 2020;30:R383–R387. doi: 10.1016/j.cub.2020.03.051. [DOI] [PubMed] [Google Scholar]
- 40.Sunyer R., Conte V., Escribano J., Elosegui-Artola A., Labernadie A., Valon L. Collective cell durotaxis emerges from long-range intercellular force transmission. Science. 2016;353:1157–1161. doi: 10.1126/science.aaf7119. [DOI] [PubMed] [Google Scholar]
- 41.Jalan R., De Chiara F., Balasubramaniyan V., Andreola F., Khetan V., Malago M. Ammonia produces pathological changes in human hepatic stellate cells and is a target for therapy of portal hypertension. J Hepatol. 2016;64:823–833. doi: 10.1016/j.jhep.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 42.Jansen C., Bogs C., Verlinden W., Thiele M., Moller P., Gortzen J. Shear-wave elastography of the liver and spleen identifies clinically significant portal hypertension: a prospective multicentre study. Liver Int. 2017;37:396–405. doi: 10.1111/liv.13243. [DOI] [PubMed] [Google Scholar]
- 43.Fernandez M. Molecular pathophysiology of portal hypertension. Hepatology. 2015;61:1406–1415. doi: 10.1002/hep.27343. [DOI] [PubMed] [Google Scholar]
- 44.Kostallari E., Shah V.H. Pericytes in the liver. Adv Exp Med Biol. 2019;1122:153–167. doi: 10.1007/978-3-030-11093-2_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corpechot C., Barbu V., Wendum D., Kinnman N., Rey C., Poupon R. Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology. 2002;35:1010–1021. doi: 10.1053/jhep.2002.32524. [DOI] [PubMed] [Google Scholar]
- 46.Fernandez M., Vizzutti F., Garcia-Pagan J.C., Rodes J., Bosch J. Anti-VEGF receptor-2 monoclonal antibody prevents portal-systemic collateral vessel formation in portal hypertensive mice. Gastroenterology. 2004;126:886–894. doi: 10.1053/j.gastro.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 47.Fernandez M., Mejias M., Garcia-Pras E., Mendez R., Garcia-Pagan J.C., Bosch J. Reversal of portal hypertension and hyperdynamic splanchnic circulation by combined vascular endothelial growth factor and platelet-derived growth factor blockade in rats. Hepatology. 2007;46:1208–1217. doi: 10.1002/hep.21785. [DOI] [PubMed] [Google Scholar]
- 48.Hennenberg M., Trebicka J., Stark C., Kohistani A.Z., Heller J., Sauerbruch T. Sorafenib targets dysregulated Rho kinase expression and portal hypertension in rats with secondary biliary cirrhosis. Br J Pharmacol. 2009;157:258–270. doi: 10.1111/j.1476-5381.2009.00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uschner F.E., Schueller F., Nikolova I., Klein S., Schierwagen R., Magdaleno F. The multikinase inhibitor regorafenib decreases angiogenesis and improves portal hypertension. Oncotarget. 2018;9:36220–36237. doi: 10.18632/oncotarget.26333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coch L., Mejias M., Berzigotti A., Garcia-Pras E., Gallego J., Bosch J. Disruption of negative feedback loop between vasohibin-1 and vascular endothelial growth factor decreases portal pressure, angiogenesis, and fibrosis in cirrhotic rats. Hepatology. 2014;60:633–647. doi: 10.1002/hep.26995. [DOI] [PubMed] [Google Scholar]
- 51.Van Steenkiste C., Geerts A., Vanheule E., Van Vlierberghe H., De Vos F., Olievier K. Role of placental growth factor in mesenteric neoangiogenesis in a mouse model of portal hypertension. Gastroenterology. 2009;137 doi: 10.1053/j.gastro.2009.08.068. 2112-2124 e2111-2116. [DOI] [PubMed] [Google Scholar]
- 52.Cole W.C., Welsh D.G. Role of myosin light chain kinase and myosin light chain phosphatase in the resistance arterial myogenic response to intravascular pressure. Arch Biochem Biophys. 2011;510:160–173. doi: 10.1016/j.abb.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 53.van Beuge M.M., Prakash J., Lacombe M., Gosens R., Post E., Reker-Smit C. Reduction of fibrogenesis by selective delivery of a Rho kinase inhibitor to hepatic stellate cells in mice. J Pharmacol Exp Ther. 2011;337:628–635. doi: 10.1124/jpet.111.179143. [DOI] [PubMed] [Google Scholar]
- 54.van Beuge M.M., Prakash J., Lacombe M., Post E., Reker-Smit C., Beljaars L. Increased liver uptake and reduced hepatic stellate cell activation with a cell-specific conjugate of the Rho-kinase inhibitor Y27632. Pharm Res. 2011;28:2045–2054. doi: 10.1007/s11095-011-0430-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sauerbruch T., Trebicka J. Future therapy of portal hypertension in liver cirrhosis - a guess. F1000prime Rep. 2014;6:95. doi: 10.12703/P6-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hennenberg M., Trebicka J., Biecker E., Schepke M., Sauerbruch T., Heller J. Vascular dysfunction in human and rat cirrhosis: role of receptor-desensitizing and calcium-sensitizing proteins. Hepatology. 2007;45:495–506. doi: 10.1002/hep.21502. [DOI] [PubMed] [Google Scholar]
- 57.Trebicka J., Hennenberg M., Schulze Probsting A., Laleman W., Klein S., Granzow M. Role of beta3-adrenoceptors for intrahepatic resistance and portal hypertension in liver cirrhosis. Hepatology. 2009;50:1924–1935. doi: 10.1002/hep.23222. [DOI] [PubMed] [Google Scholar]
- 58.Trebicka J., Leifeld L., Hennenberg M., Biecker E., Eckhardt A., Fischer N. Hemodynamic effects of urotensin II and its specific receptor antagonist palosuran in cirrhotic rats. Hepatology. 2008;47:1264–1276. doi: 10.1002/hep.22170. [DOI] [PubMed] [Google Scholar]
- 59.Kageyama Y., Ikeda H., Watanabe N., Nagamine M., Kusumoto Y., Yashiro M. Antagonism of sphingosine 1-phosphate receptor 2 causes a selective reduction of portal vein pressure in bile duct-ligated rodents. Hepatology. 2012;56:1427–1438. doi: 10.1002/hep.25780. [DOI] [PubMed] [Google Scholar]
- 60.Xu W., Lu C., Zhang F., Shao J., Zheng S. Dihydroartemisinin restricts hepatic stellate cell contraction via an FXR-S1PR2-dependent mechanism. IUBMB Life. 2016;68:376–387. doi: 10.1002/iub.1492. [DOI] [PubMed] [Google Scholar]
- 61.Moleda L., Trebicka J., Dietrich P., Gabele E., Hellerbrand C., Straub R.H. Amelioration of portal hypertension and the hyperdynamic circulatory syndrome in cirrhotic rats by neuropeptide Y via pronounced splanchnic vasoaction. Gut. 2011;60:1122–1132. doi: 10.1136/gut.2010.226407. [DOI] [PubMed] [Google Scholar]
- 62.Grace J.A., Klein S., Herath C.B., Granzow M., Schierwagen R., Masing N. Activation of the MAS receptor by angiotensin-(1-7) in the renin-angiotensin system mediates mesenteric vasodilatation in cirrhosis. Gastroenterology. 2013;145:874–884 e875. doi: 10.1053/j.gastro.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 63.Klein S., Herath C.B., Schierwagen R., Grace J., Haltenhof T., Uschner F.E. Hemodynamic effects of the non-peptidic angiotensin-(1-7) agonist AVE0991 in liver cirrhosis. PloS One. 2015;10 doi: 10.1371/journal.pone.0138732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murthy K.S. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol. 2006;68:345–374. doi: 10.1146/annurev.physiol.68.040504.094707. [DOI] [PubMed] [Google Scholar]
- 65.Uschner F.E., Gluckert K., Paternostro R., Gnad T., Schierwagen R., Mandorfer M. Combination of phosphodiesterase-5-inhibitors and beta blockers improves experimental portal hypertension and erectile dysfunction. Liver Int. 2020;40:2228–2241. doi: 10.1111/liv.14586. [DOI] [PubMed] [Google Scholar]
- 66.Schwabl P., Brusilovskaya K., Supper P., Bauer D., Konigshofer P., Riedl F. The soluble guanylate cyclase stimulator riociguat reduces fibrogenesis and portal pressure in cirrhotic rats. Sci Rep. 2018;8:9372. doi: 10.1038/s41598-018-27656-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schaffner D., Lazaro A., Deibert P., Hasselblatt P., Stoll P., Fauth L. Analysis of the nitric oxide-cyclic guanosine monophosphate pathway in experimental liver cirrhosis suggests phosphodiesterase-5 as potential target to treat portal hypertension. World J Gastroenterol. 2018;24:4356–4368. doi: 10.3748/wjg.v24.i38.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kreisel W., Schaffner D., Lazaro A., Trebicka J., Merfort I., Schmitt-Graeff A. Phosphodiesterases in the liver as potential therapeutic targets of cirrhotic portal hypertension. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21176223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li T., Eheim A.L., Klein S., Uschner F.E., Smith A.C., Brandon-Warner E. Novel role of nuclear receptor Rev-erbalpha in hepatic stellate cell activation: potential therapeutic target for liver injury. Hepatology. 2014;59:2383–2396. doi: 10.1002/hep.27049. [DOI] [PubMed] [Google Scholar]
- 70.McConnell M., Iwakiri Y. Biology of portal hypertension. Hepatol Int. 2018;12:11–23. doi: 10.1007/s12072-017-9826-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wanless I.R., Wong F., Blendis L.M., Greig P., Heathcote E.J., Levy G. Hepatic and portal vein thrombosis in cirrhosis: possible role in development of parenchymal extinction and portal hypertension. Hepatology. 1995;21:1238–1247. [PubMed] [Google Scholar]
- 72.Neubauer K., Knittel T., Armbrust T., Ramadori G. Accumulation and cellular localization of fibrinogen/fibrin during short-term and long-term rat liver injury. Gastroenterology. 1995;108:1124–1135. doi: 10.1016/0016-5085(95)90211-2. [DOI] [PubMed] [Google Scholar]
- 73.Duplantier J.G., Dubuisson L., Senant N., Freyburger G., Laurendeau I., Herbert J.M. A role for thrombin in liver fibrosis. Gut. 2004;53:1682–1687. doi: 10.1136/gut.2003.032136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marsden P.A., Ning Q., Fung L.S., Luo X., Chen Y., Mendicino M. The Fgl2/fibroleukin prothrombinase contributes to immunologically mediated thrombosis in experimental and human viral hepatitis. J Clin Invest. 2003;112:58–66. doi: 10.1172/JCI18114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abdel-Salam O.M., Baiuomy A.R., Ameen A., Hassan N.S. A study of unfractionated and low molecular weight heparins in a model of cholestatic liver injury in the rat. Pharmacol Res. 2005;51:59–67. doi: 10.1016/j.phrs.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 76.Assy N., Hussein O., Khalil A., Luder A., Szvalb S., Paizi M. The beneficial effect of aspirin and enoxaparin on fibrosis progression and regenerative activity in a rat model of cirrhosis. Dig Dis Sci. 2007;52:1187–1193. doi: 10.1007/s10620-006-9595-1. [DOI] [PubMed] [Google Scholar]
- 77.Abe W., Ikejima K., Lang T., Okumura K., Enomoto N., Kitamura T. Low molecular weight heparin prevents hepatic fibrogenesis caused by carbon tetrachloride in the rat. J Hepatol. 2007;46:286–294. doi: 10.1016/j.jhep.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 78.Cerini F., Vilaseca M., Lafoz E., Garcia-Irigoyen O., Garcia-Caldero H., Tripathi D.M. Enoxaparin reduces hepatic vascular resistance and portal pressure in cirrhotic rats. J Hepatol. 2016;64:834–842. doi: 10.1016/j.jhep.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 79.Fortea J.I., Zipprich A., Fernandez-Mena C., Puerto M., Bosoi C.R., Almagro J. Enoxaparin does not ameliorate liver fibrosis or portal hypertension in rats with advanced cirrhosis. Liver Int. 2018;38:102–112. doi: 10.1111/liv.13510. [DOI] [PubMed] [Google Scholar]
- 80.Vilaseca M., Garcia-Caldero H., Lafoz E., Garcia-Irigoyen O., Avila M.A., Reverter J.C. The anticoagulant Rivaroxaban lowers portal hypertension in cirrhotic rats mainly by deactivating hepatic stellate cells. Hepatology. 2017;65:2031–2044. doi: 10.1002/hep.29084. [DOI] [PubMed] [Google Scholar]
- 81.Simonetto D.A., Yang H.Y., Yin M., de Assuncao T.M., Kwon J.H., Hilscher M. Chronic passive venous congestion drives hepatic fibrogenesis via sinusoidal thrombosis and mechanical forces. Hepatology. 2015;61:648–659. doi: 10.1002/hep.27387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hilscher M.B., Sehrawat T., Arab J.P., Zeng Z., Gao J., Liu M. Mechanical stretch increases expression of CXCL1 in liver sinusoidal endothelial cells to recruit neutrophils, generate sinusoidal microthombi, and promote portal hypertension. Gastroenterology. 2019;157:193–209 e199. doi: 10.1053/j.gastro.2019.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bosch J., Iwakiri Y. The portal hypertension syndrome: etiology, classification, relevance, and animal models. Hepatol Int. 2018;12:1–10. doi: 10.1007/s12072-017-9827-9. [DOI] [PubMed] [Google Scholar]
- 84.Lam K.C., Juttner H.U., Reynolds T.B. Spontaneous portosystemic shunt: relationship to spontaneous encephalopathy and gastrointestinal hemorrhage. Dig Dis Sci. 1981;26:346–352. doi: 10.1007/BF01308377. [DOI] [PubMed] [Google Scholar]
- 85.Sumanovski L.T., Battegay E., Stumm M., van der Kooij M., Sieber C.C. Increased angiogenesis in portal hypertensive rats: role of nitric oxide. Hepatology. 1999;29:1044–1049. doi: 10.1002/hep.510290436. [DOI] [PubMed] [Google Scholar]
- 86.Iwakiri Y., Shah V., Rockey D.C. Vascular pathobiology in chronic liver disease and cirrhosis - current status and future directions. J Hepatol. 2014;61:912–924. doi: 10.1016/j.jhep.2014.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tiani C., Garcia-Pras E., Mejias M., de Gottardi A., Berzigotti A., Bosch J. Apelin signaling modulates splanchnic angiogenesis and portosystemic collateral vessel formation in rats with portal hypertension. J Hepatol. 2009;50:296–305. doi: 10.1016/j.jhep.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 88.Huang H.C., Wang S.S., Hsin I.F., Chang C.C., Lee F.Y., Lin H.C. Cannabinoid receptor 2 agonist ameliorates mesenteric angiogenesis and portosystemic collaterals in cirrhotic rats. Hepatology. 2012;56:248–258. doi: 10.1002/hep.25625. [DOI] [PubMed] [Google Scholar]
- 89.Mejias M., Garcia-Pras E., Tiani C., Miquel R., Bosch J., Fernandez M. Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology. 2009;49:1245–1256. doi: 10.1002/hep.22758. [DOI] [PubMed] [Google Scholar]
- 90.Reiberger T., Angermayr B., Schwabl P., Rohr-Udilova N., Mitterhauser M., Gangl A. Sorafenib attenuates the portal hypertensive syndrome in partial portal vein ligated rats. J Hepatol. 2009;51:865–873. doi: 10.1016/j.jhep.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 91.Van Steenkiste C., Ribera J., Geerts A., Pauta M., Tugues S., Casteleyn C. Inhibition of placental growth factor activity reduces the severity of fibrosis, inflammation, and portal hypertension in cirrhotic mice. Hepatology. 2011;53:1629–1640. doi: 10.1002/hep.24238. [DOI] [PubMed] [Google Scholar]
- 92.Hassan M., Moghadamrad S., Sorribas M., Muntet S.G., Kellmann P., Trentesaux C. Paneth cells promote angiogenesis and regulate portal hypertension in response to microbial signals. J Hepatol. 2020;73:628–639. doi: 10.1016/j.jhep.2020.03.019. [DOI] [PubMed] [Google Scholar]
- 93.Garcia-Pagan J.C., Gracia-Sancho J., Bosch J. Functional aspects on the pathophysiology of portal hypertension in cirrhosis. J Hepatol. 2012;57:458–461. doi: 10.1016/j.jhep.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 94.Wiest R., Das S., Cadelina G., Garcia-Tsao G., Milstien S., Groszmann R.J. Bacterial translocation in cirrhotic rats stimulates eNOS-derived NO production and impairs mesenteric vascular contractility. J Clin Invest. 1999;104:1223–1233. doi: 10.1172/JCI7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tazi K.A., Barriere E., Moreau R., Heller J., Sogni P., Pateron D. Role of shear stress in aortic eNOS up-regulation in rats with biliary cirrhosis. Gastroenterology. 2002;122:1869–1877. doi: 10.1053/gast.2002.33586. [DOI] [PubMed] [Google Scholar]
- 96.Tazi K.A., Moreau R., Herve P., Dauvergne A., Cazals-Hatem D., Bert F. Norfloxacin reduces aortic NO synthases and proinflammatory cytokine up-regulation in cirrhotic rats: role of Akt signaling. Gastroenterology. 2005;129:303–314. doi: 10.1053/j.gastro.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 97.Queck A., Carnevale R., Uschner F.E., Schierwagen R., Klein S., Jansen C. Role of portal venous platelet activation in patients with decompensated cirrhosis and TIPS. Gut. 2020;69:1535–1536. doi: 10.1136/gutjnl-2019-319044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Violi F., Ferro D., Basili S., Lionetti R., Rossi E., Merli M. Ongoing prothrombotic state in the portal circulation of cirrhotic patients. Thromb Haemost. 1997;77:44–47. [PubMed] [Google Scholar]
- 99.Benten D., Schulze zur Wiesch J., Sydow K., Koops A., Buggisch P., Boger R.H. The transhepatic endotoxin gradient is present despite liver cirrhosis and is attenuated after transjugular portosystemic shunt (TIPS) BMC Gastroenterol. 2011;11:107. doi: 10.1186/1471-230X-11-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hennenberg M., Biecker E., Trebicka J., Jochem K., Zhou Q., Schmidt M. Defective RhoA/Rho-kinase signaling contributes to vascular hypocontractility and vasodilation in cirrhotic rats. Gastroenterology. 2006;130:838–854. doi: 10.1053/j.gastro.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 101.Casey S., Schierwagen R., Mak K.Y., Klein S., Uschner F., Jansen C. Activation of the alternate renin-angiotensin system correlates with the clinical status in human cirrhosis and corrects post liver transplantation. J Clin Med. 2019;8 doi: 10.3390/jcm8040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Klein S., Van Beuge M.M., Granzow M., Beljaars L., Schierwagen R., Kilic S. HSC-specific inhibition of Rho-kinase reduces portal pressure in cirrhotic rats without major systemic effects. J Hepatol. 2012;57:1220–1227. doi: 10.1016/j.jhep.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 103.Klein S., Frohn F., Magdaleno F., Reker-Smit C., Schierwagen R., Schierwagen I. Rho-kinase inhibitor coupled to peptide-modified albumin carrier reduces portal pressure and increases renal perfusion in cirrhotic rats. Sci Rep. 2019;9:2256. doi: 10.1038/s41598-019-38678-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schmidt T.S.B., Raes J., Bork P. The human gut microbiome: from association to modulation. Cell. 2018;172:1198–1215. doi: 10.1016/j.cell.2018.02.044. [DOI] [PubMed] [Google Scholar]
- 105.Tilg H., Cani P.D., Mayer E.A. Gut microbiome and liver diseases. Gut. 2016;65:2035–2044. doi: 10.1136/gutjnl-2016-312729. [DOI] [PubMed] [Google Scholar]
- 106.De Gottardi A., Rautou P.E., Schouten J., Rubbia-Brandt L., Leebeek F., Trebicka J. Porto-sinusoidal vascular disease: proposal and description of a novel entity. Lancet Gastroenterol Hepatol. 2019;4:399–411. doi: 10.1016/S2468-1253(19)30047-0. [DOI] [PubMed] [Google Scholar]
- 107.Albillos A., de Gottardi A., Rescigno M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J Hepatol. 2020;72:558–577. doi: 10.1016/j.jhep.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 108.Garcia-Tsao G., Albillos A., Barden G.E., West A.B. Bacterial translocation in acute and chronic portal hypertension. Hepatology. 1993;17:1081–1085. [PubMed] [Google Scholar]
- 109.Garcia-Tsao G., Lee F.Y., Barden G.E., Cartun R., West A.B. Bacterial translocation to mesenteric lymph nodes is increased in cirrhotic rats with ascites. Gastroenterology. 1995;108:1835–1841. doi: 10.1016/0016-5085(95)90147-7. [DOI] [PubMed] [Google Scholar]
- 110.Sorribas M., Jakob M.O., Yilmaz B., Li H., Stutz D., Noser Y. FXR modulates the gut-vascular barrier by regulating the entry sites for bacterial translocation in experimental cirrhosis. J Hepatol. 2019;71:1126–1140. doi: 10.1016/j.jhep.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 111.Jansen C., Chatterjee D.A., Thomsen K.L., Al-Kassou B., Sawhney R., Jones H. Significant reduction in heart rate variability is a feature of acute decompensation of cirrhosis and predicts 90-day mortality. Aliment Pharmacol Ther. 2019;50:568–579. doi: 10.1111/apt.15365. [DOI] [PubMed] [Google Scholar]
- 112.Wiest R., Lawson M., Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60:197–209. doi: 10.1016/j.jhep.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 113.Wiest R., Albillos A., Trauner M., Bajaj J.S., Jalan R. Targeting the gut-liver axis in liver disease. J Hepatol. 2017;67:1084–1103. doi: 10.1016/j.jhep.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 114.Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bajaj J.S., Khoruts A. Microbiota changes and intestinal microbiota transplantation in liver diseases and cirrhosis. J Hepatol. 2020;72:1003–1027. doi: 10.1016/j.jhep.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 116.Tranah T.H., Edwards L.A., Schnabl B., Shawcross D.L. Targeting the gut-liver-immune axis to treat cirrhosis. Gut. 2020 doi: 10.1136/gutjnl-2020-320786. [DOI] [PubMed] [Google Scholar]
- 117.Verbeke L., Farre R., Trebicka J., Komuta M., Roskams T., Klein S. Obeticholic acid, a farnesoid X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats. Hepatology. 2014;59:2286–2298. doi: 10.1002/hep.26939. [DOI] [PubMed] [Google Scholar]
- 118.Verbeke L., Farre R., Verbinnen B., Covens K., Vanuytsel T., Verhaegen J. The FXR agonist obeticholic acid prevents gut barrier dysfunction and bacterial translocation in cholestatic rats. Am J Pathol. 2015;185:409–419. doi: 10.1016/j.ajpath.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 119.Verbeke L., Mannaerts I., Schierwagen R., Govaere O., Klein S., Vander Elst I. FXR agonist obeticholic acid reduces hepatic inflammation and fibrosis in a rat model of toxic cirrhosis. Sci Rep. 2016;6:33453. doi: 10.1038/srep33453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ubeda M., Lario M., Munoz L., Borrero M.J., Rodriguez-Serrano M., Sanchez-Diaz A.M. Obeticholic acid reduces bacterial translocation and inhibits intestinal inflammation in cirrhotic rats. J Hepatol. 2016;64:1049–1057. doi: 10.1016/j.jhep.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 121.Munoz L., Borrero M.J., Ubeda M., Conde E., Del Campo R., Rodriguez-Serrano M. Intestinal immune dysregulation driven by dysbiosis promotes barrier disruption and bacterial translocation in rats with cirrhosis. Hepatology. 2019;70:925–938. doi: 10.1002/hep.30349. [DOI] [PubMed] [Google Scholar]
- 122.Tyc O., Jansen C., Schierwagen R., Uschner F.E., Israelsen M., Klein S. Variation in bile microbiome by the etiology of cholestatic liver disease. Liver Transpl. 2020 doi: 10.1002/lt.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schierwagen R., Alvarez-Silva C., Madsen M.S.A., Kolbe C.C., Meyer C., Thomas D. Circulating microbiome in blood of different circulatory compartments. Gut. 2019;68:578–580. doi: 10.1136/gutjnl-2018-316227. [DOI] [PubMed] [Google Scholar]
- 124.Alvarez-Silva C., Schierwagen R., Pohlmann A., Magdaleno F., Uschner F.E., Ryan P. Compartmentalization of immune response and microbial translocation in decompensated cirrhosis. Front Immunol. 2019;10:69. doi: 10.3389/fimmu.2019.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jansen C., Moller P., Meyer C., Kolbe C.C., Bogs C., Pohlmann A. Increase in liver stiffness after transjugular intrahepatic portosystemic shunt is associated with inflammation and predicts mortality. Hepatology. 2018;67:1472–1484. doi: 10.1002/hep.29612. [DOI] [PubMed] [Google Scholar]
- 126.Czaja A.J., Carpenter H.A. Decreased fibrosis during corticosteroid therapy of autoimmune hepatitis. J Hepatol. 2004;40:646–652. doi: 10.1016/j.jhep.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 127.Marcellin P., Gane E., Buti M., Afdhal N., Sievert W., Jacobson I.M. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–475. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 128.Mandorfer M., Kozbial K., Schwabl P., Freissmuth C., Schwarzer R., Stern R. Sustained virologic response to interferon-free therapies ameliorates HCV-induced portal hypertension. J Hepatol. 2016;65:692–699. doi: 10.1016/j.jhep.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 129.Trebicka J., Fernandez J., Papp M., Caraceni P., Laleman W., Gambino C. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J Hepatol. 2020;73:842–854. doi: 10.1016/j.jhep.2020.06.013. [DOI] [PubMed] [Google Scholar]
- 130.Martinez J., Hernandez-Gea V., Rodriguez-de-Santiago E., Tellez L., Procopet B., Giraldez A. Bacterial infections in patients with acute variceal bleeding in the era of antibiotic prophylaxis. J Hepatol. 2021 doi: 10.1016/j.jhep.2021.03.026. [DOI] [PubMed] [Google Scholar]
- 131.Bosch J., Abraldes J.G., Berzigotti A., Garcia-Pagan J.C. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol. 2009;6:573–582. doi: 10.1038/nrgastro.2009.149. [DOI] [PubMed] [Google Scholar]
- 132.Rossle M. TIPS: 25 years later. J Hepatol. 2013;59:1081–1093. doi: 10.1016/j.jhep.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 133.Lai L., Poneros J., Santilli J., Brugge W. EUS-guided portal vein catheterization and pressure measurement in an animal model: a pilot study of feasibility. Gastrointest Endosc. 2004;59:280–283. doi: 10.1016/s0016-5107(03)02544-6. [DOI] [PubMed] [Google Scholar]
- 134.Huang J.Y., Samarasena J.B., Tsujino T., Lee J., Hu K.Q., McLaren C.E. EUS-guided portal pressure gradient measurement with a simple novel device: a human pilot study. Gastrointest Endosc. 2017;85:996–1001. doi: 10.1016/j.gie.2016.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ryou M., Stylopoulos N. Endoscopic ultrasound-guided sampling and profiling of portal circulation in human patients for metabolic research studies and biomarker assessment. Am J Physiol Gastrointest Liver Physiol. 2020;319:G584–G588. doi: 10.1152/ajpgi.00135.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Berzigotti A., Seijo S., Arena U., Abraldes J.G., Vizzutti F., Garcia-Pagan J.C. Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology. 2013;144:102–111 e101. doi: 10.1053/j.gastro.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 137.de Franchis R., Baveno V.I.F. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–752. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 138.Jansen C., Bogs C., Verlinden W., Thiele M., Moller P., Gortzen J. Algorithm to rule out clinically significant portal hypertension combining Shear-wave elastography of liver and spleen: a prospective multicentre study. Gut. 2016;65:1057–1058. doi: 10.1136/gutjnl-2016-311536. [DOI] [PubMed] [Google Scholar]
- 139.Thiele M., Hugger M.B., Kim Y., Rautou P.E., Elkrief L., Jansen C. 2D shear wave liver elastography by Aixplorer to detect portal hypertension in cirrhosis: an individual patient data meta-analysis. Liver Int. 2020;40:1435–1446. doi: 10.1111/liv.14439. [DOI] [PubMed] [Google Scholar]
- 140.Pavlides M., Banerjee R., Sellwood J., Kelly C.J., Robson M.D., Booth J.C. Multiparametric magnetic resonance imaging predicts clinical outcomes in patients with chronic liver disease. J Hepatol. 2016;64:308–315. doi: 10.1016/j.jhep.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Luetkens J.A., Klein S., Traber F., Schmeel F.C., Sprinkart A.M., Kuetting D.L.R. Quantification of liver fibrosis at T1 and T2 mapping with extracellular volume fraction MRI: preclinical results. Radiology. 2018;288:748–754. doi: 10.1148/radiol.2018180051. [DOI] [PubMed] [Google Scholar]
- 142.Luetkens J.A., Klein S., Traeber F., Schmeel F.C., Sprinkart A.M., Kuetting D.L.R. Quantitative liver MRI including extracellular volume fraction for non-invasive quantification of liver fibrosis: a prospective proof-of-concept study. Gut. 2018;67:593–594. doi: 10.1136/gutjnl-2017-314561. [DOI] [PubMed] [Google Scholar]
- 143.Mesropyan N., Isaak A., Faron A., Praktiknjo M., Jansen C., Kuetting D. Magnetic resonance parametric mapping of the spleen for non-invasive assessment of portal hypertension. Eur Radiol. 2020 doi: 10.1007/s00330-020-07080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Leeming D.J., Veidal S.S., Karsdal M.A., Nielsen M.J., Trebicka J., Busk T. Pro-C5, a marker of true type V collagen formation and fibrillation, correlates with portal hypertension in patients with alcoholic cirrhosis. Scand J Gastroenterol. 2015;50:584–592. doi: 10.3109/00365521.2014.996590. [DOI] [PubMed] [Google Scholar]
- 145.Jansen C., Leeming D.J., Mandorfer M., Byrjalsen I., Schierwagen R., Schwabl P. PRO-C3-levels in patients with HIV/HCV-Co-infection reflect fibrosis stage and degree of portal hypertension. PloS One. 2014;9 doi: 10.1371/journal.pone.0108544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Praktiknjo M., Lehmann J., Nielsen M.J., Schierwagen R., Uschner F.E., Meyer C. Acute decompensation boosts hepatic collagen type III deposition and deteriorates experimental and human cirrhosis. Hepatol Commun. 2018;2:211–222. doi: 10.1002/hep4.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Trebicka J., Krag A., Gansweid S., Appenrodt B., Schiedermaier P., Sauerbruch T. Endotoxin and tumor necrosis factor-receptor levels in portal and hepatic vein of patients with alcoholic liver cirrhosis receiving elective transjugular intrahepatic portosystemic shunt. Eur J Gastroenterol Hepatol. 2011;23:1218–1225. doi: 10.1097/MEG.0b013e32834a75dc. [DOI] [PubMed] [Google Scholar]
- 148.Trebicka J., Krag A., Gansweid S., Schiedermaier P., Strunk H.M., Fimmers R. Soluble TNF-alpha-receptors I are prognostic markers in TIPS-treated patients with cirrhosis and portal hypertension. PloS One. 2013;8 doi: 10.1371/journal.pone.0083341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Monteiro S., Grandt J., Uschner F.E., Kimer N., Madsen J.L., Schierwagen R. Differential inflammasome activation predisposes to acute-on-chronic liver failure in human and experimental cirrhosis with and without previous decompensation. Gut. 2020 doi: 10.1136/gutjnl-2019-320170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Praktiknjo M., Monteiro S., Grandt J., Kimer N., Madsen J.L., Werge M.P. Cardiodynamic state is associated with systemic inflammation and fatal acute-on-chronic liver failure. Liver Int. 2020;40:1457–1466. doi: 10.1111/liv.14433. [DOI] [PubMed] [Google Scholar]
- 151.Trebicka J., Anadol E., Elfimova N., Strack I., Roggendorf M., Viazov S. Hepatic and serum levels of miR-122 after chronic HCV-induced fibrosis. J Hepatol. 2013;58:234–239. doi: 10.1016/j.jhep.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 152.Jansen C., Eischeid H., Goertzen J., Schierwagen R., Anadol E., Strassburg C.P. The role of miRNA-34a as a prognostic biomarker for cirrhotic patients with portal hypertension receiving TIPS. PloS One. 2014;9 doi: 10.1371/journal.pone.0103779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jansen C., Reiberger T., Huang J., Eischeid H., Schierwagen R., Mandorfer M. Circulating miRNA-122 levels are associated with hepatic necroinflammation and portal hypertension in HIV/HCV coinfection. PloS One. 2015;10 doi: 10.1371/journal.pone.0116768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Abraldes J.G., Trebicka J., Chalasani N., D'Amico G., Rockey D.C., Shah V.H. Prioritization of therapeutic targets and trial design in cirrhotic portal hypertension. Hepatology. 2019;69:1287–1299. doi: 10.1002/hep.30314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lebrec D., Nouel O., Corbic M., Benhamou J.P. Propranolol--a medical treatment for portal hypertension? Lancet. 1980;2:180–182. doi: 10.1016/s0140-6736(80)90063-x. [DOI] [PubMed] [Google Scholar]
- 156.Lebrec D., Hillon P., Munoz C., Goldfarb G., Nouel O., Benhamou J.P. The effect of propranolol on portal hypertension in patients with cirrhosis: a hemodynamic study. Hepatology. 1982;2:523–527. doi: 10.1002/hep.1840020502. [DOI] [PubMed] [Google Scholar]
- 157.Tripathi D., Ferguson J.W., Kochar N., Leithead J.A., Therapondos G., McAvoy N.C. Multicenter randomized controlled trial of carvedilol versus variceal band ligation for the prevention of the first variceal bleed. Hepatology. 2007;46 doi: 10.1002/hep.23045. 269a-269a. [DOI] [PubMed] [Google Scholar]
- 158.Tripathi D., Ferguson J.W., Kochar N., Leithead J.A., Therapondos G., McAvoy N.C. Randomized controlled trial of carvedilol versus variceal band ligation for the prevention of the first variceal bleed. Hepatology. 2009;50:825–833. doi: 10.1002/hep.23045. [DOI] [PubMed] [Google Scholar]
- 159.Reiberger T., Ulbrich G., Ferlitsch A., Payer B.A., Schwabl P., Pinter M. Carvedilol for primary prophylaxis of variceal bleeding in cirrhotic patients with haemodynamic non-response to propranolol. Gut. 2013;62:1634–1641. doi: 10.1136/gutjnl-2012-304038. [DOI] [PubMed] [Google Scholar]
- 160.Villanueva C., Albillos A., Genesca J., Garcia-Pagan J.C., Calleja J.L., Aracil C. Beta blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2019;393:1597–1608. doi: 10.1016/S0140-6736(18)31875-0. [DOI] [PubMed] [Google Scholar]
- 161.Groszmann R.J., Garcia-Tsao G., Bosch J., Grace N.D., Burroughs A.K., Planas R. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353:2254–2261. doi: 10.1056/NEJMoa044456. [DOI] [PubMed] [Google Scholar]
- 162.Krag A., Wiest R., Albillos A., Gluud L.L. The window hypothesis: haemodynamic and non-haemodynamic effects of beta-blockers improve survival of patients with cirrhosis during a window in the disease. Gut. 2012;61:967–969. doi: 10.1136/gutjnl-2011-301348. [DOI] [PubMed] [Google Scholar]
- 163.Trebicka J. Emergency TIPS in a Child-Pugh B patient: when does the window of opportunity open and close? J Hepatol. 2017;66:442–450. doi: 10.1016/j.jhep.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 164.European Association for the Study of the L EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–460. doi: 10.1016/j.jhep.2018.03.024. European Association for the Study of the Liver. Electronic address eee. [DOI] [PubMed] [Google Scholar]
- 165.Wong F. Recent advances in our understanding of hepatorenal syndrome. Nat Rev Gastroenterol Hepatol. 2012;9:382–391. doi: 10.1038/nrgastro.2012.96. [DOI] [PubMed] [Google Scholar]
- 166.Reiberger T., Ferlitsch A., Payer B.A., Mandorfer M., Heinisch B.B., Hayden H. Non-selective betablocker therapy decreases intestinal permeability and serum levels of LBP and IL-6 in patients with cirrhosis. J Hepatol. 2013;58:911–921. doi: 10.1016/j.jhep.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 167.Rossle M., Haag K., Ochs A., Sellinger M., Noldge G., Perarnau J.M. The transjugular intrahepatic portosystemic stent-shunt procedure for variceal bleeding. N Engl J Med. 1994;330:165–171. doi: 10.1056/NEJM199401203300303. [DOI] [PubMed] [Google Scholar]
- 168.Brensing K.A., Textor J., Perz J., Schiedermaier P., Raab P., Strunk H. Long term outcome after transjugular intrahepatic portosystemic stent-shunt in non-transplant cirrhotics with hepatorenal syndrome: a phase II study. Gut. 2000;47:288–295. doi: 10.1136/gut.47.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tan H.K., James P.D., Sniderman K.W., Wong F. Long-term clinical outcome of patients with cirrhosis and refractory ascites treated with transjugular intrahepatic portosystemic shunt insertion. J Gastroenterol Hepatol. 2015;30:389–395. doi: 10.1111/jgh.12725. [DOI] [PubMed] [Google Scholar]
- 170.Trebicka J. Does transjugular intrahepatic portosystemic shunt stent differentially improve survival in a subset of cirrhotic patients? Semin Liver Dis. 2018;38:87–96. doi: 10.1055/s-0038-1627457. [DOI] [PubMed] [Google Scholar]
- 171.Fiorucci S., Antonelli E., Morelli O., Mencarelli A., Casini A., Mello T. NCX-1000, a NO-releasing derivative of ursodeoxycholic acid, selectively delivers NO to the liver and protects against development of portal hypertension. Proc Natl Acad Sci U S A. 2001;98:8897–8902. doi: 10.1073/pnas.151136298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Berzigotti A., Bellot P., De Gottardi A., Garcia-Pagan J.C., Gagnon C., Spenard J. NCX-1000, a nitric oxide-releasing derivative of UDCA, does not decrease portal pressure in patients with cirrhosis: results of a randomized, double-blind, dose-escalating study. Am J Gastroenterol. 2010;105:1094–1101. doi: 10.1038/ajg.2009.661. [DOI] [PubMed] [Google Scholar]
- 173.Kalambokis G.N., Mouzaki A., Rodi M., Pappas K., Fotopoulos A., Xourgia X. Rifaximin improves systemic hemodynamics and renal function in patients with alcohol-related cirrhosis and ascites. Clin Gastroenterol Hepatol. 2012;10:815–818. doi: 10.1016/j.cgh.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 174.Kimer N., Pedersen J.S., Busk T.M., Gluud L.L., Hobolth L., Krag A. Rifaximin has no effect on hemodynamics in decompensated cirrhosis: a randomized, double-blind, placebo-controlled trial. Hepatology. 2017;65:592–603. doi: 10.1002/hep.28898. [DOI] [PubMed] [Google Scholar]
- 175.Eguchi A., Koyama Y., Wree A., Johnson C.D., Nakamura R., Povero D. Emricasan, a pan-caspase inhibitor, improves survival and portal hypertension in a murine model of common bile-duct ligation. J Mol Med (Berl) 2018;96:575–583. doi: 10.1007/s00109-018-1642-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gracia-Sancho J., Manicardi N., Ortega-Ribera M., Maeso-Diaz R., Guixe-Muntet S., Fernandez-Iglesias A. Emricasan ameliorates portal hypertension and liver fibrosis in cirrhotic rats through a hepatocyte-mediated paracrine mechanism. Hepatol Commun. 2019;3:987–1000. doi: 10.1002/hep4.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Garcia-Tsao G., Fuchs M., Shiffman M., Borg B.B., Pyrsopoulos N., Shetty K. Emricasan (IDN-6556) lowers portal pressure in patients with compensated cirrhosis and severe portal hypertension. Hepatology. 2019;69:717–728. doi: 10.1002/hep.30199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Garcia-Tsao G., Bosch J., Kayali Z., Harrison S.A., Abdelmalek M.F., Lawitz E. Randomized placebo-controlled trial of emricasan for non-alcoholic steatohepatitis-related cirrhosis with severe portal hypertension. J Hepatol. 2020;72:885–895. doi: 10.1016/j.jhep.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 179.Trebicka J., Hennenberg M., Laleman W., Shelest N., Biecker E., Schepke M. Atorvastatin lowers portal pressure in cirrhotic rats by inhibition of RhoA/Rho-kinase and activation of endothelial nitric oxide synthase. Hepatology. 2007;46:242–253. doi: 10.1002/hep.21673. [DOI] [PubMed] [Google Scholar]
- 180.Trebicka J., Hennenberg M., Odenthal M., Shir K., Klein S., Granzow M. Atorvastatin attenuates hepatic fibrosis in rats after bile duct ligation via decreased turnover of hepatic stellate cells. J Hepatol. 2010;53:702–712. doi: 10.1016/j.jhep.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 181.Abraldes J.G., Albillos A., Banares R., Turnes J., Gonzalez R., Garcia-Pagan J.C. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: a randomized controlled trial. Gastroenterology. 2009;136:1651–1658. doi: 10.1053/j.gastro.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 182.Pollo-Flores P., Soldan M., Santos U.C., Kunz D.G., Mattos D.E., da Silva A.C. Three months of simvastatin therapy vs. placebo for severe portal hypertension in cirrhosis: a randomized controlled trial. Dig Liver Dis. 2015;47:957–963. doi: 10.1016/j.dld.2015.07.156. [DOI] [PubMed] [Google Scholar]
- 183.Abraldes J.G., Villanueva C., Aracil C., Turnes J., Hernandez-Guerra M., Genesca J. Addition of simvastatin to standard therapy for the prevention of variceal rebleeding does not reduce rebleeding but increases survival in patients with cirrhosis. Gastroenterology. 2016;150:1160–1170 e1163. doi: 10.1053/j.gastro.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 184.Pose E., Napoleone L., Amin A., Campion D., Jimenez C., Piano S. Safety of two different doses of simvastatin plus rifaximin in decompensated cirrhosis (LIVERHOPE-SAFETY): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Gastroenterol Hepatol. 2020;5:31–41. doi: 10.1016/S2468-1253(19)30320-6. [DOI] [PubMed] [Google Scholar]
- 185.Rodriguez S., Raurell I., Torres-Arauz M., Garcia-Lezana T., Genesca J., Martell M. A nitric oxide-donating statin decreases portal pressure with a better toxicity profile than conventional statins in cirrhotic rats. Sci Rep. 2017;7:40461. doi: 10.1038/srep40461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Verbeke L., Farre R., Covens K., Verbinnen BV T., Elst I.V., Windmolders PV J. Obeticholic acid, a Farnesoid-X Receptor-agonist, reduces bacterial translocation and restores intestinal permeability in a rat model of cholestatic liver disease. J Hepatol. 2014;60:S1–S22. [Google Scholar]
- 187.Schwabl P., Hambruch E., Seeland B.A., Hayden H., Wagner M., Garnys L. The FXR agonist PX20606 ameliorates portal hypertension by targeting vascular remodelling and sinusoidal dysfunction. J Hepatol. 2017;66:724–733. doi: 10.1016/j.jhep.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 188.Semmler G., Simbrunner B., Scheiner B., Schwabl P., Paternostro R., Bucsics T. Impact of farnesoid X receptor single nucleotide polymorphisms on hepatic decompensation and mortality in cirrhotic patients with portal hypertension. J Gastroenterol Hepatol. 2019;34:2164–2172. doi: 10.1111/jgh.14700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Villa E., Camma C., Marietta M., Luongo M., Critelli R., Colopi S. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology. 2012;143:1253–1260 e1254. doi: 10.1053/j.gastro.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 190.Serper M., Weinberg E.M., Cohen J.B., Reese P.P., Taddei T.H., Kaplan D.E. Mortality and hepatic decompensation in patients with cirrhosis and atrial fibrillation treated with anticoagulation. Hepatology. 2020 doi: 10.1002/hep.31264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fallowfield J.A., Hayden A.L., Snowdon V.K., Aucott R.L., Stutchfield B.M., Mole D.J. Relaxin modulates human and rat hepatic myofibroblast function and ameliorates portal hypertension in vivo. Hepatology. 2014;59:1492–1504. doi: 10.1002/hep.26627. [DOI] [PubMed] [Google Scholar]
- 192.Snowdon V.K., Lachlan N.J., Hoy A.M., Hadoke P.W., Semple S.I., Patel D. Serelaxin as a potential treatment for renal dysfunction in cirrhosis: preclinical evaluation and results of a randomized phase 2 trial. PloS Med. 2017;14 doi: 10.1371/journal.pmed.1002248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gifford F.J., Dunne P.D.J., Weir G., Ireland H., Graham C., Tuck S. A phase 2 randomised controlled trial of serelaxin to lower portal pressure in cirrhosis (STOPP) Trials. 2020;21:260. doi: 10.1186/s13063-020-4203-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rodriguez-Vilarrupla A., Lavina B., Garcia-Caldero H., Russo L., Rosado E., Roglans N. PPARalpha activation improves endothelial dysfunction and reduces fibrosis and portal pressure in cirrhotic rats. J Hepatol. 2012;56:1033–1039. doi: 10.1016/j.jhep.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 195.Schwabl P., Payer B.A., Grahovac J., Klein S., Horvatits T., Mitterhauser M. Pioglitazone decreases portosystemic shunting by modulating inflammation and angiogenesis in cirrhotic and non-cirrhotic portal hypertensive rats. J Hepatol. 2014;60:1135–1142. doi: 10.1016/j.jhep.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 196.Tsai H.C., Li T.H., Huang C.C., Huang S.F., Liu R.S., Yang Y.Y. Beneficial effects of the peroxisome proliferator-activated receptor alpha/gamma agonist aleglitazar on progressive hepatic and splanchnic abnormalities in cirrhotic rats with portal hypertension. Am J Pathol. 2018;188:1608–1624. doi: 10.1016/j.ajpath.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 197.Kemp W., Kompa A., Phrommintikul A., Herath C., Zhiyuan J., Angus P. Urotensin II modulates hepatic fibrosis and portal hemodynamic alterations in rats. Am J Physiol Gastrointest Liver Physiol. 2009;297:G762–G767. doi: 10.1152/ajpgi.00127.2009. [DOI] [PubMed] [Google Scholar]
- 198.Zhang R., Chen J., Liu D., Wang Y. Urotensin II receptor antagonist reduces hepatic resistance and portal pressure through enhanced eNOS-dependent HSC vasodilatation in CCl4-induced cirrhotic rats. Front Med. 2019;13:398–408. doi: 10.1007/s11684-019-0689-5. [DOI] [PubMed] [Google Scholar]
- 199.Granzow M., Schierwagen R., Klein S., Kowallick B., Huss S., Linhart M. Angiotensin-II type 1 receptor-mediated Janus kinase 2 activation induces liver fibrosis. Hepatology. 2014;60:334–348. doi: 10.1002/hep.27117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Klein S., Rick J., Lehmann J., Schierwagen R., Schierwagen I.G., Verbeke L. Janus-kinase-2 relates directly to portal hypertension and to complications in rodent and human cirrhosis. Gut. 2017;66:145–155. doi: 10.1136/gutjnl-2015-309600. [DOI] [PubMed] [Google Scholar]
- 201.Wang D., Yin J., Dong R., Zhao J., Wang Q., Wang N. Inhibition of Janus kinase-2 signalling pathway ameliorates portal hypertensive syndrome in partial portal hypertensive and liver cirrhosis rats. Dig Liver Dis. 2015;47:315–323. doi: 10.1016/j.dld.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 202.Wang D., Wang Q., Yin J., Dong R., Wang Q., Du X. Combined administration of propranolol + AG490 offers better effects on portal hypertensive rats with cirrhosis. J Gastroenterol Hepatol. 2016;31:1037–1044. doi: 10.1111/jgh.13207. [DOI] [PubMed] [Google Scholar]
- 203.Klein S., Kleine C.E., Pieper A., Granzow M., Gautsch S., Himmit M. TGR(mREN2)27 rats develop non-alcoholic fatty liver disease-associated portal hypertension responsive to modulations of Janus-kinase 2 and Mas receptor. Sci Rep. 2019;9:11598. doi: 10.1038/s41598-019-48024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tan H.K., Leow W.Q., Chang P.E. Ruxolitinib for the treatment of portal hypertension in a patient with primary myelofibrosis. Gastroenterology. 2019;157:e26–e27. doi: 10.1053/j.gastro.2016.08.059. [DOI] [PubMed] [Google Scholar]
- 205.Brensing K.A., Horsch M., Textor J., Schiedermaier P., Raab P., Schepke M. Hemodynamic effects of propranolol and nitrates in cirrhotics with transjugular intrahepatic portosystemic stent-shunt. Scand J Gastroenterol. 2002;37:1070–1076. doi: 10.1080/003655202320378284. [DOI] [PubMed] [Google Scholar]
- 206.Vasina V., Giannone F., Domenicali M., Latorre R., Berzigotti A., Caraceni P. Portal hypertension and liver cirrhosis in rats: effect of the beta3-adrenoceptor agonist SR58611A. Br J Pharmacol. 2012;167:1137–1147. doi: 10.1111/j.1476-5381.2012.02074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Eden E., Navon R., Steinfeld I., Lipson D., Yakhini Z. GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinformatics. 2009;10:48. doi: 10.1186/1471-2105-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Supek F., Bosnjak M., Skunca N., Smuc T. REVIGO summarizes and visualizes long lists of gene ontology terms. PloS One. 2011;6 doi: 10.1371/journal.pone.0021800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.