Fig. 4.
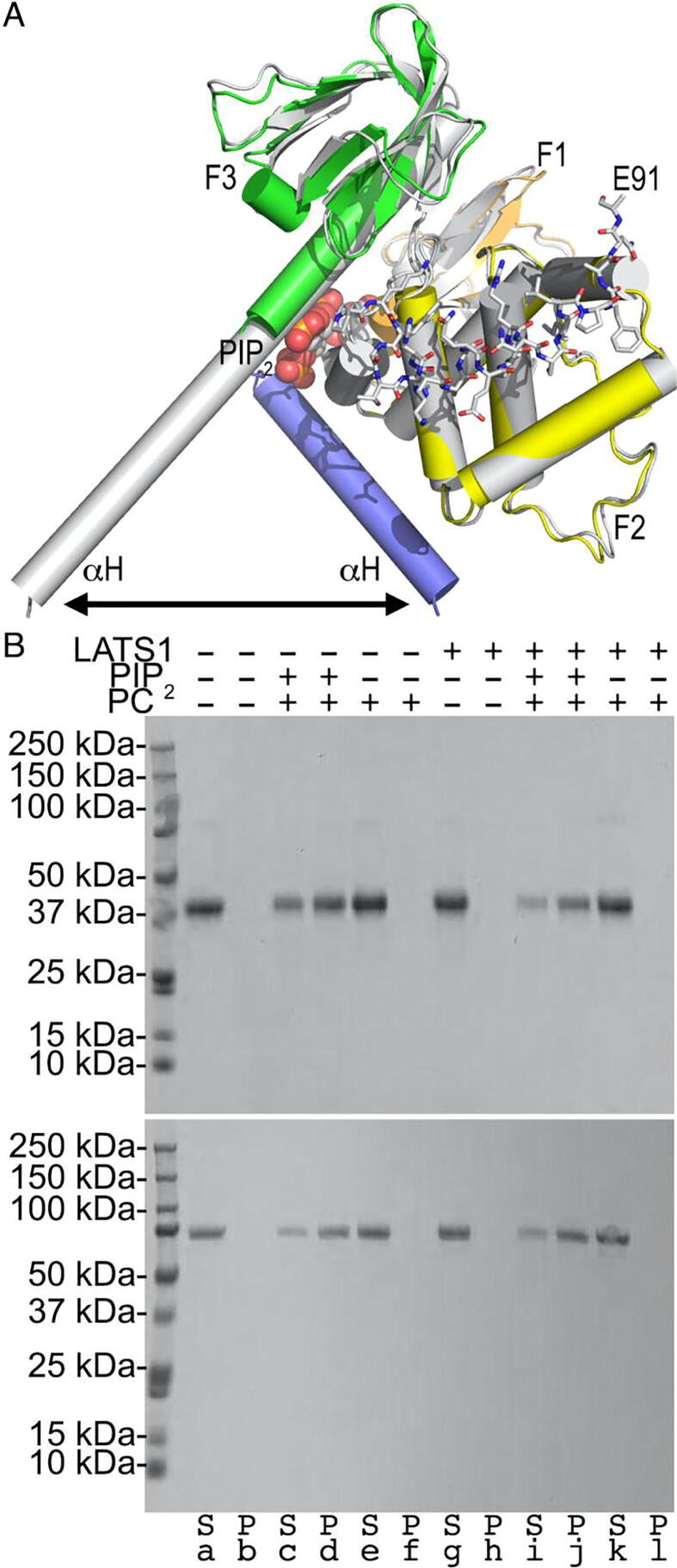
LASTS1 binding is independent of Nf2/merlin attachment to the membrane. A. Superposition of our LATS1-bound Nf2/merlin structure (FERM domain: F1, orange; F2, yellow; F3, green; the first α-helix αH of the central helical domain, blue) in complex with LATS1 shown in stick representation onto the Nf2/merlin (gray) domain in complex with PIP2 (PDB entry 6cds) [62]. The LATS1 C-terminus, Nf2/merlin FERM subdomains, and PIP2 are labeled. The double arrow indicates the movement of the α-helix (αH) that extends upon activation. B. Lipid co-sedimentation assay of the Nf2/merlin head domain (top) and of full-length Nf2/merlin (bottom) as analyzed by sodium dodecyl sulfate–polyacrylamide electrophoresis gel. Nf2/merlin (top, head domain, residues 1–339; bottom full-length) does not pellet in the absence of liposomes (lanes a and b) or with phosphatidylcholine (PC) liposomes (lanes e and f) or when bound to LATS1 (lanes g and h) alone or in the presence of PC liposomes (lanes k and l). Both Nf2/merlin proteins (top and bottom) bind to phosphatidylinositol 4,5-bisphosphate (PIP2) containing liposomes in the absence (lanes d) or presence of LATS1 (lanes j). S, supernatant; P, pellet. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)