Figure 2. B cells directly modulate the MHC-II expression on CCR2− myocardial macrophages.
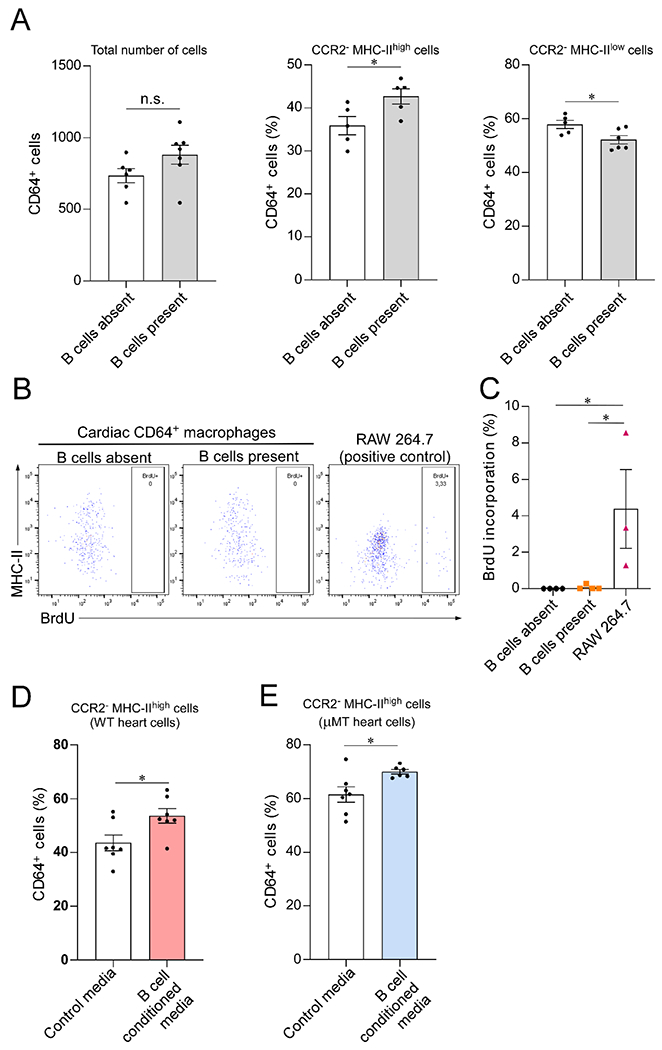
(A) Cardiac CD64+ macrophages isolated from μMT hearts cultured alone or in the presence of WT CD19+ splenic B cells. Coculture with B cells increased the prevalence of CCR2−MHC-IIhigh macrophages. Left, total number of CD64+ macrophages analyzed by flow cytometry after 40 hours in culture (n= 6 and 7 samples/condition; p=0.1). Middle, CCR2−MHC-IIhigh macrophages in relation to the total number of cells (n= 5 samples/condition). Right, CCR2− MHC-IIlow macrophages in relation to the total number of cells (n= 5 and 6 samples/condition). (B) Representative flow cytometry plots representing the expression of MHC-II and BrdU incorporation by cardiac CD64+ macrophages cocultured with B cells (n= 4 samples/condition). RAW 264.7 macrophage-derived cells were used as a positive control for BrdU incorporation (n=3 samples/condition). (C) Bar graph showing that BrdU incorporation by cultured primary cardiac CD64+ macrophages is close to zero and is not affected by the presence of B cells. (D-E) Exposure to B cell conditioned media increased the percentage of CCR2−MHC-IIhigh cells in primary cardiac macrophages isolated from both WT (n = 7 samples per condition) and μMT animals (n = 7 and 6 samples/condition), suggesting that the effect of B cells is, at least in part, mediated by a secreted molecules. Outliers defined as experimental points two standard deviations away from the mean were excluded. Statistical comparisons between two groups were performed using two-tailed Student’s t-test (A, D and E) or One-Way ANOVA followed by Tukey’s test for multiple comparison (C). Bars represent mean ± SEM. n.s. = not significant; *p<0.05.
