Abstract
Background
Transcatheter aortic valve replacement (TAVR) is now a common procedure to treat and improve quality of life, clinical outcomes, and self-sufficiency in high-risk patients with aortic stenosis, and its use has been expanding rapidly in younger and low-risk populations. The aim of this study was to evaluate the outcomes, trends, and predictors of major bleeding in patients undergoing TAVR.
Methodology
We utilized the National Inpatient Sample (NIS) data from the year 2015 to 2018. International Classification of Disease 10 codes were utilized to extract data. Baseline characteristics were compared using Pearson’s chi-square test for categorical variables and independent samples t-test for continuous variables. A multivariable logistic regression model was used to evaluate the predictors of major bleeding. Propensity matching was done for adjusted analysis to compare outcomes in TAVR with and without major bleeding. The outcomes of interest in this study were (1) predictors of major bleeding after TAVR; (2) in-hospital mortality; and (3) resource utilization in terms of cost and length of stay.
Results
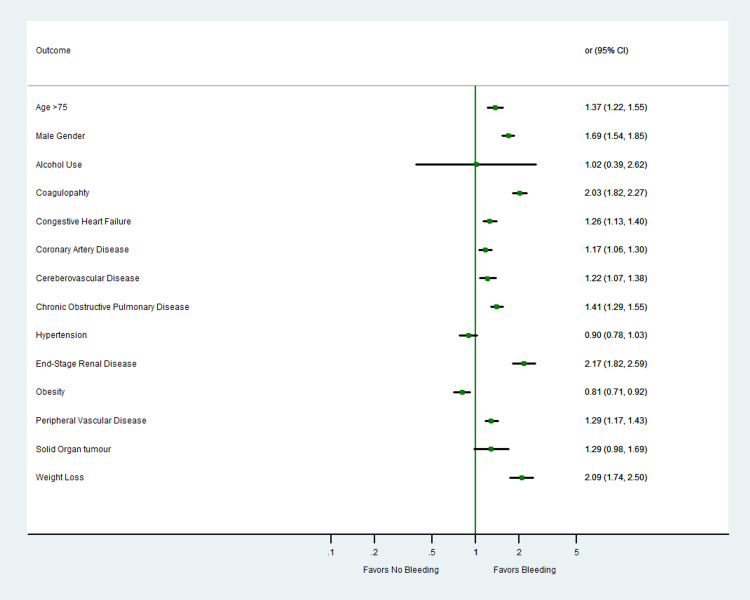
A total of 34,752 weighted hospitalizations for TAVR were included in the analysis. Of the patients undergoing the procedure, 2,294 (6.6%) had a major bleed while 32,458 (93.3%) did not. At baseline, patients with coagulopathy (odds ratio [OR]: 2.03; 95% confidence interval [CI]: 1.82-2.27), congestive heart failure (OR: 1.26; 95% CI: 1.13-1.40), chronic obstructive pulmonary disease (OR: 1.41; 95% CI: 1.29-1.55), liver disease (OR: 1.96; 95% CI: 1.61-2.39), peripheral vascular disease (OR: 1.29; 95% CI: 1.17-1.43), cerebrovascular disease (OR: 1.22; 95% CI: 1.07-1.38), end-stage renal disease (ESRD) (OR: 2.17; 95% CI: 1.82-2.59), and coronary artery disease (OR: 1.17; 95% Cl: 1.06-1.30) had higher adjusted rates of odds of major bleeding. Patients who had major bleeding had a higher median cost of stay (US$60,326 vs. US$45490) and length of stay (seven vs. three days).
Conclusions
Mortality is higher in patients with major bleeding, and at baseline, coagulopathy and ESRD are significant predictors of a major bleed in patients undergoing TAVR.
Keywords: transcatheter aortic valve implantation, transcatheter aortic valve replacement, tavr, tavi, aortic stenosis, major bleeding
Introduction
In the medium-to-high-risk elderly patients who suffer from severe aortic stenosis and calcified aortic valve disease, transcatheter aortic valve replacement (TAVR) has proven to be an effective and minimally invasive procedure [1,2]. The three landmark PARTNER trials have exhaustively evaluated the efficacy and side effect profile of TAVR which has stood the test of time [2,3]. It is an effective alternative to surgical valve replacement and has been widely adopted in the United States [3,4].
According to the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry investigators, TAVR has been reported to have a 92% procedural success rate [5]. In patients with aortic stenosis, TAVR has led to constant improvement in clinical outcomes along with improved techniques as the procedure has undergone significant evolution given increased operator experience [6,7].
Major bleeding is a life-threatening complication of surgical aortic valve replacement (10% surgical aortic valve replacement [SAVR] versus 6% for TAVR) [1]. However, it is important to mention that despite complication rates being lower with TAVR compared to the surgical option, major bleeding remains a significant complication identified in the landmark PARTNER trials [1-3].
One of the most frequent complications post-TAVR with the first PARTNER trial was major bleeding which reported 10.2% bleeding events [8]. Major bleed is known to be associated with significant mortality and morbidity even though it is less frequent compared to SAVR [9,10]. Post-TAVR, major bleeding was reported to increase one-month mortality by 32% [11]. Data on outcomes of TAVR patients who develop major bleeding remain limited. Hence, the main objective of this study was to comprehensively evaluate the predictors and outcomes of major bleeding in TAVR using the National Inpatient Sample (NIS) database.
Materials and methods
The NIS database was used to identify cases of TAVR performed during 2015-2018. NIS is a publicly available database and does not contain any patient-sensitive information. Hence, this study did not require ethical board approval or informed consent [12]. NIS data are collected annually and are representative of 20% of the hospitalizations in the United States. The cost of hospitalization is also recorded in US dollars which comprises the cost incurred in return for services provided.
National analysis was performed using the international classification of diseases (ICD)-9 (3505, 3506), and ICD-10 (02RF3) codes were utilized to identify all cases of TAVR. We extracted cases from all available procedure fields. Patients younger than 18 years of age were not included in the analysis. Data on comorbidities are provided by the NIS. Major bleeding in our analysis was defined as any bleeding requiring transfusion. The outcomes of interest for our study were (1) predictors of major bleeding after TAVR; (2) in-hospital mortality; and (3) resource utilization in terms of cost and length of stay.
We used the discharge weights provided by NIS to perform analysis on weighted hospitalizations. We used the Mann-Whitney U test for all continuous variables as they are not normally distributed, and the chi-square test was used for categorical variables. To test for the non-normality of data we used the Shapiro-Wilk test. Entry method was used to develop a binary logistic model including baseline comorbidities such as obesity, weight loss, metastatic cancer, lymphoma, solid organ tumor, alcohol use, coagulopathy, hypothyroidism, chronic obstructive pulmonary disease (COPD), cerebrovascular disease (CVA), congestive heart failure (CHF), coronary artery disease (CAD), diabetes mellitus, hypertension, liver disease, chronic kidney disease (CKD), and peripheral vascular disease (PVD). Demographic factors such as age, sex, race, median income, and hospital location were also included in the analysis. Observations with less than 11 cases were not reported in compliance with the Health Cost and Utilization Project. R software version 3.5 was used for all analyses. We considered a p-value of <0.05 to be statistically significant.
Results
A total of 34,752 weighted hospitalizations for TAVR were included in the analysis. Of the patients undergoing the procedure, 2,294 (6.6%) had bleeding complications while 32,458 (93.3%) did not. The detailed baseline characteristics are summarized in Table 1. At baseline, patients with coagulopathy (odds ratio [OR]: 2.03; 95% confidence interval [CI]: 1.82-2.27), CHF (OR: 1.26; 95% CI: 1.13-1.40), COPD (OR: 1.41; 95% CI: 1.29-1.55), liver disease (OR: 1.96; 95% CI: 1.61-2.39), PVD (OR: 1.29; 95% CI: 1.17-1.43), CVA (OR: 1.22; 95% CI: 1.07-1.38), end-stage renal disease (ESRD) (OR: 2.17; 95% CI: 1.82-2.59), and CAD (OR: 1.17; 95% Cl: 1.06-1.30) had higher adjusted rates of odds of major bleeding (Table 1; Figure 1). Patients who had major bleeding had a higher median cost of stay (US$60,326 vs. US$45,490) and length of stay (seven vs. three days) (Table 2).
Table 1. Baseline characteristics and predictors of major bleeding in patients after TAVR.
OR: odds ratio; CI: confidence interval; IQR: interquartile range; CHF: congestive heart failure; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; ESRD: end-stage renal disease; PVD: peripheral vascular disease; TAVR: transcatheter aortic valve replacement
| Multivariate analysis OR (95% CI) | |||
| Variable. (%) | Without major bleed (32,458) | With major bleed (2,294) | No bleeding vs. bleeding |
| Age, median (IQR) | 81 (75-86) | 82 (76-87) | 1.37 (1.22-1.53) |
| Female gender | 14,823 (45.7%) | 1,290 (56.2%) | 1.69 (1.54-1.85) |
| Caucasian | 27,107 (87.2%) | 1,828 (82.6%) | Reference |
| African Americans | 1,267 (4.1%) | 146 (6.6%) | 1.53 (1.27-1.86) |
| Hispanics | 1,480 (4.8%) | 148 (6.7%) | 1.48 (1.24-1.78) |
| Alcohol use | 52 (0.2%) | 5 (0.2%) | 1.02 (0.39-2.62) |
| Hypothyroidism | 6,591 (20.3%) | 489 (21.3%) | 0.96 (0.86-1.07) |
| Coagulopathy | 3,792 (11.7%) | 525 (22.9%) | 2.03 (1.82-2.27) |
| CHF | 23,725 (73.1%) | 1,793 (78.2%) | 1.26 (1.13-1.40) |
| CAD | 22,458 (69.2%) | 1,627 (70.9%) | 1.17 (1.06-1.30) |
| Cerebrovascular disease | 3,711 (11.4%) | 339 (14.8%) | 1.22 (1.07-1.38) |
| COPD | 9,785 (30.1%) | 863 (37.6%) | 1.41 (1.29-1.55) |
| Diabetes mellitus | 5,144 (15.8%) | 282 (12.3%) | 0.85 (0.75-0.98) |
| Hypertension | 28,886 (89.0%) | 2,038 (88.8%) | 0.90 (0.78-1.03) |
| Liver disease | 949 (2.9%) | 144 (6.3%) | 1.96 (1.61-2.39) |
| ESRD | 1,128 (3.5%) | 186 (8.1%) | 2.17 (1.82-2.59) |
| Obesity | 5,545 (17.1%) | 296 (12.9%) | 0.81 (0.71-0.92) |
| PVD | 7,000 (21.6%) | 629 (27.4%) | 1.29 (1.17-1.43) |
| Weight loss | 979 (3.0%) | 168 (7.3%) | 2.09 (1.74-2.50) |
| Metastatic cancer | 210 (0.6%) | 19 (0.8%) | 1.20 (0.71-2.03) |
| Lymphoma | 221 (0.7%) | 16 (0.7%) | 1.21 (0.72-2.03) |
| Solid organ tumor | 780 (2.4%) | 72 (3.1%) | 1.29 (0.98-1.69) |
| Income 0-25th percentile | 6,838 (21.4%) | 481 (21.3%) | Reference |
| 25th–50th | 8,255 (25.8%) | 493 (21.8%) | 0.92 (0.80-1.05) |
| 50th–75th | 8,544 (26.7%) | 608 (26.9%) | 1.11 (0.98-1.27) |
| 75th–100th | 8,336 (26.1%) | 677 (30.0%) | 1.26 (1.11-1.44) |
| Urban | 277 (0.9%) | 21 (0.9%) | Reference |
| Urban nonteaching | 2,997 (9.2%) | 238 (10.4%) | 1.09 (0.66-1.81) |
| Urban teaching | 29,184 (89.9%) | 2,035 (88.7%) | 0.91 (0.56-1.48) |
Table 2. In-hospital outcomes of patients with and without bleed in TAVR.
TAVR: transcatheter aortic valve replacement
| Outcome | No bleed | Bleed | P-value |
| Died during hospitalization | 432 (1.3%) | 146 (6.4%) | <0.01 |
| Median length of stay (days) | 3 (2-5) | 7 (4-13) | <0.01 |
| Median cost of stay (US$) | 45,490 (35,640-57,665) | 60,326 (45,756-81,222) | <0.01 |
Figure 1. Adjusted odds of predictors of in-hospital major bleeding in patients undergoing TAVR.
TAVR: transcatheter aortic valve replacement
Discussion
Approximately 6% of patients who undergo TAVR have major bleeding. Of the patients who have major bleeding, 6.4% die during hospitalization. We also identified important baseline characteristics such as age greater than 75, female sex, and history of ESRD, liver disease, PVD, CHF, and CAD.
Major bleeding or vascular complications are expected to decrease as TAVR technology evolves into smaller device sizes [13,14]. However, bleeding complications have been underreported and are inconsistent in the early literature [10]. Patients who undergo TAVR are typically frail, elderly, and are at a risk for both bleeding and ischemic complications [12,15,16]. Careful risk and benefit evaluation is warranted to identify the antithrombotic regimen, as major late bleeding complications are not only frequent but also associated with an increased risk of total mortality [17]. With baseline hematological problems, higher bleeding complications related to coagulation factors and platelets were identified in our study. According to the literature, our findings are reinforced by a good association between blood disorders and major bleeding events [16].
According to a previous study, major bleeding is associated with a three-fold increase in one-year mortality following TAVR and SAVR [18]. A previous study reported that major bleeding and life-threatening bleeding after TAVR, as defined by the Valve Academic Research Consortium (VARC) criteria, occurred in approximately 15-20% of TAVR procedures [13]. Factors that increase the risk of bleeding include a high prevalence of CKD, peripheral vasculopathy, acquired thrombocytopenia, and acquired reversible von Willebrand factor deficiency [16,19-21]. It is important to note that our definition of major bleeding was not based on well-validated VARC-II criteria similar to previous studies, instead, we used ICD codes to define major bleeding. However, our study findings are in agreement with prior reported data as we report higher bleeding risk with PVD (1.29 times) and coagulopathy (2.03 times).
After the procedure, patients undergoing TAVR had lower rates of major or life-threatening bleeding (11.3% vs. 20.9%), acute kidney injury (AKI) stage II and III, and cardiogenic shock compared to those undergoing SAVR [13,20-26]. Due to the inherent platelet dysfunction, patients with ESRD have a propensity to bleed and are associated with high mortality [19,20]. Similarly, due to associated coagulopathy, liver disease patients have an increased tendency to bleed [27]. In a previous meta-analysis, patients with chronic liver disease had a higher incidence of bleeding complications, need for blood transfusions, and mortality, which was further exacerbated by antiplatelet drug use [28,29]. As reported by Tchetche et al., 38.9% of patients undergoing TAVR received at least one transfusion [10,13,19,29]. Our estimate of major bleeding complications in TAVR was 6.6%, which we believe to be a more contemporary estimate. Moreover, we report 2.17 and 1.96 times higher odds of bleeding with ESRD and liver disease, respectively. Interestingly, our study found that obesity and hypertension are associated with a lower risk of bleeding. The phenomenon of the obesity paradox has been seen in our study, which was previously described for diseases such as myocardial infarction, heart failure, and renal disease [30]. Similarly, prior TAVR literature data based on NIS reported hypertension as a protective factor against mortality [18].
NIS provides an opportunity to identify characteristics from a large sample size to evaluate the predictors. For instance, previously, it was found that peptic ulcer disease and colon cancer lead to high gastrointestinal bleeding after TAVR. This led to a change in the recommendation to perform a pre-TAVR colonoscopy and proton pump inhibitor use to mitigate the risk. Similarly, patients undergoing TAVR who were found to have a high risk of AKI underwent pre-TAVR intravenous hydration along with minimal contrast use during the procedure. Our study aimed to replicate this by providing data on these predictors so that mitigation measures can be taken against bleeding.
Our study findings are not without limitations. For instance, we could not extract data on antiplatelet and anticoagulant use as they are not available in the NIS database. Similarly, data on the cause of death are not available in the NIS. Moreover, NIS coding errors cannot be completely ruled out.
Conclusions
We report that at baseline, ESRD, liver disease, PVD, CHF, CAD, age greater than 75, and female gender are associated with a higher risk of bleeding after TAVR. It is of utmost importance to identify patients who are at a high risk of developing complications as TAVR expands to a rapidly aging population.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Principles of TAVR valve design, modelling, and testing. Rotman OM, Bianchi M, Ghosh RP, Kovarovic B, Bluestein D. Expert Rev Med Devices. 2018;15:771–791. doi: 10.1080/17434440.2018.1536427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. Mack MJ, Leon MB, Thourani VH, et al. N Engl J Med. 2019;380:1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 3.2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Otto CM, Nishimura RA, Bonow RO, et al. Circulation. 2021;143:0–71. doi: 10.1161/CIR.0000000000000932. [DOI] [PubMed] [Google Scholar]
- 4.Clinical outcomes of renal and liver transplant patients undergoing transcatheter aortic valve replacement: analysis of national inpatient sample database. Ullah W, Sattar Y, Al-Khadra Y, et al. Expert Rev Cardiovasc Ther. 2021;19:363–368. doi: 10.1080/14779072.2021.1892489. [DOI] [PubMed] [Google Scholar]
- 5.Acute kidney injury after transcatheter aortic valve replacement in the elderly: outcomes and risk management. Zaleska-Kociecka M, Dabrowski M, Stepinska J. Clin Interv Aging. 2019;14:195–201. doi: 10.2147/CIA.S149916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.How to make the TAVI pathway more efficient. Tchetche D, de Biase C, Brochado B, Mastrokostopoulos A. Interv Cardiol. 2019;14:31–33. doi: 10.15420/icr.2018.28.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abstract 17383: comparative analysis of TAVR with PCI and SAVR with CABG - a Nationwide Inpatient Sample database. Ullah W, Saleem S, Sattar Y, et al. Circulation. 2020;142:17383. [Google Scholar]
- 8.Transcatheter versus surgical aortic-valve replacement in high-risk patients. Smith CR, Leon MB, Mack MJ, et al. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 9.Adverse impact of bleeding and transfusion on the outcome post-transcatheter aortic valve implantation: insights from the Pooled-RotterdAm-Milano-Toulouse In Collaboration Plus (PRAGMATIC Plus) initiative. Tchetche D, Van der Boon RM, Dumonteil N, et al. Am Heart J. 2012;164:402–409. doi: 10.1016/j.ahj.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Clinical outcomes after transcatheter aortic valve replacement using valve academic research consortium definitions: a weighted meta-analysis of 3,519 patients from 16 studies. Généreux P, Head SJ, Van Mieghem NM, et al. J Am Coll Cardiol. 2012;59:2317–2326. doi: 10.1016/j.jacc.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Risk factors for post-TAVI bleeding according to the VARC-2 bleeding definition and effect of the bleeding on short-term mortality: a meta-analysis. Wang J, Yu W, Jin Q, Li Y, Liu N, Hou X, Yu Y. Can J Cardiol. 2017;33:525–534. doi: 10.1016/j.cjca.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Overview of the National (Nationwide) Inpatient Sample (NIS) [Jan;2021 ]; https://www.hcup-us.ahrq.gov/nisoverview.jsp#about 2019
- 13.Transcatheter versus surgical aortic valve replacement in low-risk patients. Kolte D, Vlahakes GJ, Palacios IF, Sakhuja R, Passeri JJ, Inglessis I, Elmariah S. J Am Coll Cardiol. 2019;74:1532–1540. doi: 10.1016/j.jacc.2019.06.076. [DOI] [PubMed] [Google Scholar]
- 14.A controlled trial of rivaroxaban after transcatheter aortic-valve replacement. Dangas GD, Tijssen JGP, Wöhrle J, et al. N Engl J Med. 2020;382:120–129. doi: 10.1056/NEJMoa1911425. [DOI] [PubMed] [Google Scholar]
- 15.Thrombotic versus bleeding risk after transcatheter aortic valve replacement: JACC review topic of the week. Mangieri A, Montalto C, Poletti E, et al. J Am Coll Cardiol. 2019;74:2088–2101. doi: 10.1016/j.jacc.2019.08.1032. [DOI] [PubMed] [Google Scholar]
- 16.Dual antiplatelet therapy after TAVR: a drop in the bucket? Ferlini M, Mauri S, Rossini R. Int J Cardiol. 2019;280:46–48. doi: 10.1016/j.ijcard.2019.01.069. [DOI] [PubMed] [Google Scholar]
- 17.Frailty and bleeding in older adults undergoing TAVR or SAVR: insights from the FRAILTY-AVR study. Bendayan M, Messas N, Perrault LP, et al. JACC Cardiovasc Interv. 2020;13:1058–1068. doi: 10.1016/j.jcin.2020.01.238. [DOI] [PubMed] [Google Scholar]
- 18.Predictors of in-hospital mortality in patients with end-stage renal disease undergoing transcatheter aortic valve replacement: A nationwide inpatient sample database analysis [In Press] Ullah W, Jafar M, Zahid S, et al. Cardiovasc Revasc Med. 2021 doi: 10.1016/j.carrev.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Trends and predictors of transcatheter aortic valve implantation related in-hospital mortality (From the National Inpatient Sample Database) Ullah W, Zahid S, Hamzeh I, Birnbaum Y, Virani SS, Alam M. Am J Cardiol. 2021;143:97–103. doi: 10.1016/j.amjcard.2020.12.031. [DOI] [PubMed] [Google Scholar]
- 20.Predictors of acute kidney injury after transcatheter aortic valve implantation (From National Inpatient Sample [2011-2018]) [In Press] Zahid S, Ullah W, Khan MU, Salama A, Krupica T, Khan MZ. https://doi.org/10.1016/j.amjcard.2021.04.003. Am J Cardiol. 2021 doi: 10.1016/j.amjcard.2021.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Meta-analysis comparing results of transcatheter versus surgical aortic-valve replacement in patients with severe aortic stenosis. Zhang X, Wang T, Lan R, et al. Am J Cardiol. 2020;125:449–458. doi: 10.1016/j.amjcard.2019.10.057. [DOI] [PubMed] [Google Scholar]
- 22.Transcatheter versus surgical aortic valve replacement in low and intermediate risk patients with severe aortic stenosis: systematic review and meta-analysis of randomized controlled trials and propensity score matching observational studies. Fu J, Popal MS, Li Y, et al. J Thorac Dis. 2019;11:1945–1962. doi: 10.21037/jtd.2019.04.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Assessing the safety and efficacy of TAVR compared to SAVR in low-to-intermediate surgical risk patients with aortic valve stenosis: an overview of reviews. Mc Morrow R, Kriza C, Urbán P, et al. Int J Cardiol. 2020;314:43–53. doi: 10.1016/j.ijcard.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Transcatheter versus surgical aortic valve replacement in patients at low surgical risk: a meta-analysis of randomized trials and propensity score matched observational studies. Witberg G, Lador A, Yahav D, Kornowski R. Catheter Cardiovasc Interv. 2018;92:408–416. doi: 10.1002/ccd.27518. [DOI] [PubMed] [Google Scholar]
- 25.Predictors of permanent pacemaker implantation in patients undergoing transcatheter aortic valve replacement - a systematic review and meta-analysis. Ullah W, Zahid S, Zaidi R, et al. J Am Coll Cardiol. 2021;77:981. doi: 10.1161/JAHA.121.020906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis: 1-year results from the all-comers NOTION randomized clinical trial. Thyregod HG, Steinbrüchel DA, Ihlemann N, et al. J Am Coll Cardiol. 2015;65:2184–2194. doi: 10.1016/j.jacc.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Platelets in liver and renal disease. Lambert MP. Hematology Am Soc Hematol Educ Program. 2016;2016:251–255. doi: 10.1182/asheducation-2016.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Transcatheter aortic valve implantation in the patients with chronic liver disease: a mini-review and meta-analysis. Ma X, Zhao D, Li J, et al. https://pubmed.ncbi.nlm.nih.gov/32311980. Medicine (Baltimore) 2020;99:0. doi: 10.1097/MD.0000000000019766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Comparison of two antiplatelet therapy strategies in patients undergoing transcatheter aortic valve implantation. Durand E, Blanchard D, Chassaing S, et al. Am J Cardiol. 2014;113:355–360. doi: 10.1016/j.amjcard.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 30.The "obesity paradox" explained. Banack HR, Kaufman JS. Epidemiology. 2013;24:461–462. doi: 10.1097/EDE.0b013e31828c776c. [DOI] [PubMed] [Google Scholar]