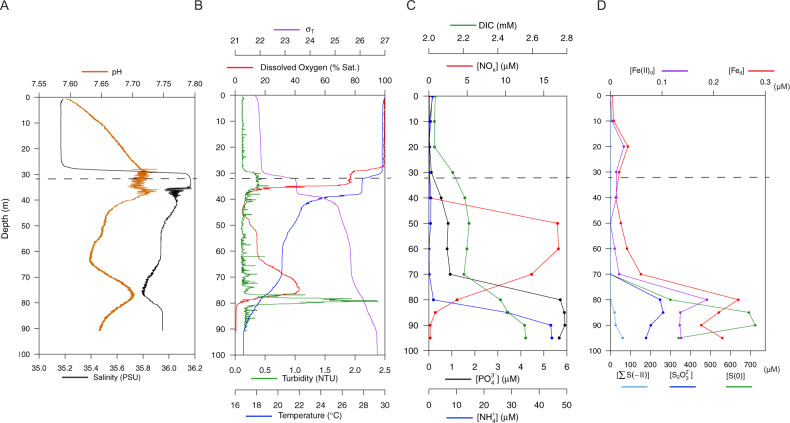
Fig. 2. The blue hole water column in September 2019 was highly stratified, with physical and chemical differences starting at the rim (at 32 m, indicated by a dashed line).
A Compared to the overlying water, salinity was slightly higher and pH was slightly lower inside the hole (i.e., below 30 m). A coincident dip in salinity and rise in pH was present at 75 m. B Dissolved oxygen concentrations varied widely, with both a primary and secondary oxycline. At 80 m, the onset of anoxia immediately below the secondary oxycline coincided with a spike in turbidity. Water density is represented by σT, defined as ρ(S,T)-1000 kg m−3 where ρ(S,T) is the density of a sample of seawater at temperature T and salinity S, measured in kg m−3, at standard atmospheric pressure. C Dissolved inorganic carbon (DIC) increased slightly from 20 to 50 m but more intensely between 70 and 90 m, from ~2.2 to 2.5 mM. A sharp increase in NOx (NO2− + NO3−) between 40 and 50 m was followed by a return to near 0 between 60 and 90 m. Phosphate (PO43−) and ammonium (NH4+) remained below 1 µm before increasing to 5–6 µm between 70 and 80 m, respectively. D Dissolved ferrous iron (Fe(II)d) and total dissolved iron (Fed) increased with the transition to anoxia. Sulfur species are presented as follows: S2O32− (thiosulfate, S in the +II oxidation state). S(0) represents combined dissolved and colloidal elemental sulfur measured after sample acidification; however, the dissolved fraction may also include a small amount of S(0) derived from the acid-dissociations of polysulfide species (i.e., Sx2−). Finally, ∑S(−II) represents primarily hydrogen sulfide (HS−) removed by acidification but could also be minorly redundant with HS− released by acidification of Sx2−. Thiosulfate peaked between 80 and 90 m, and all iron and sulfur species increased sharply by 70–85 m, with S(0) representing the largest component of the reduced sulfur pool.