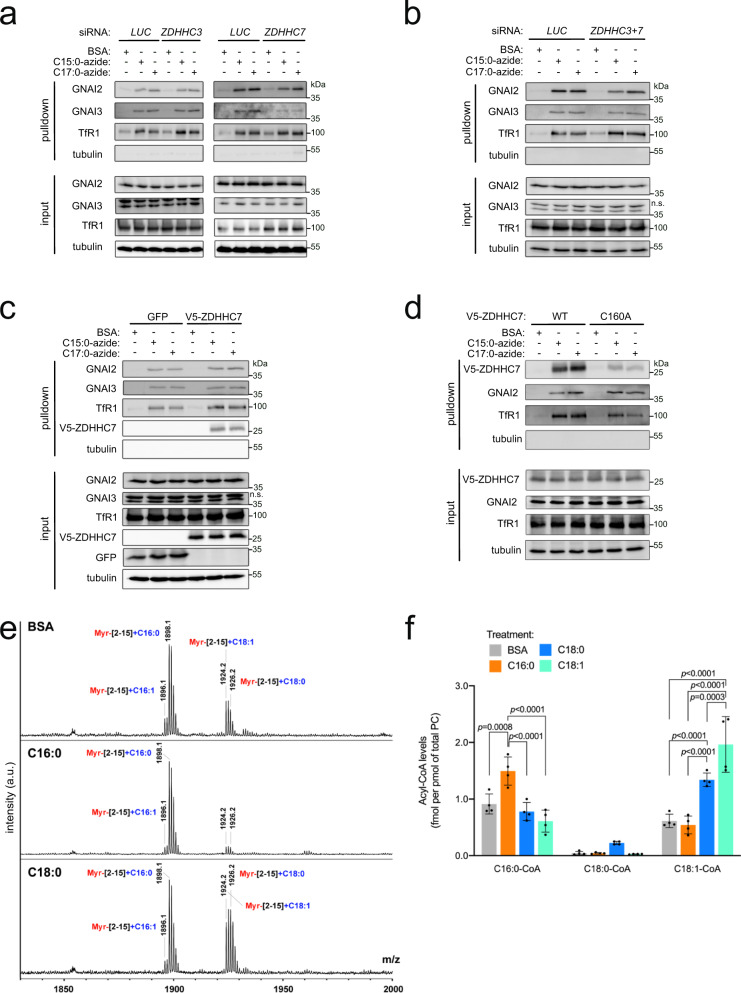
Fig. 3. The stoichiometry of GNAI3 palmitoylation versus oleoylation depends on the levels of the lipids the cells are exposed to.
a, b GNAI2 and GNAI3 are S-acylated with C15:0- and C17:0-azide by multiple acyltransferase enzymes in a redundant fashion. GNAI2 and GNAI3 acylation are only mildly reduced upon siRNA-mediated knockdown of ZDHHC7 (a), and still acylated upon combined knockdown of ZDHHC3 and ZDHHC7 (b). Metabolic labeling with 100 μM azido fatty acids was performed as in Fig. 1. Representative of three biological replicates. c Overexpression of V5-ZDHHC7 does not shift the balance in S-acylation of GNAI2 and GNAI3 proteins with C15:0- versus C17:0-azide. Metabolic labeling with 100 μM azido fatty acids was performed as in Fig. 1. Representative of four biological replicates. d V5-ZDHHC7 is modified by both C15:0- and C17:0-azide. Mutation of the active-site cysteine (C160A) blunts this acylation. Metabolic labeling with 100 μM azido fatty acids was performed as in Fig. 1. Representative of two biological replicates. e Exposure of cells to either C16:0 or C18:0 in the medium increases the fraction of GNAI protein that is either palmitoylated or oleoylated, respectively. Endogenous S-acylation of GNAI3-GFP was analyzed as in Fig. 2c in cells treated with different unlabeled fatty acids (100 μM for 3 h). f Exposure of cells to different fatty acids in the medium (100 μM for 3 h) shifts the intracellular pool of acyl-CoAs toward the corresponding fatty acid. Acyl-CoA levels were normalized to the total phosphatidyl choline (PC) content. Data are presented as mean ± SD of four biological replicates and the p-values were calculated by two-way ANOVA followed by Tukey’s post hoc test.