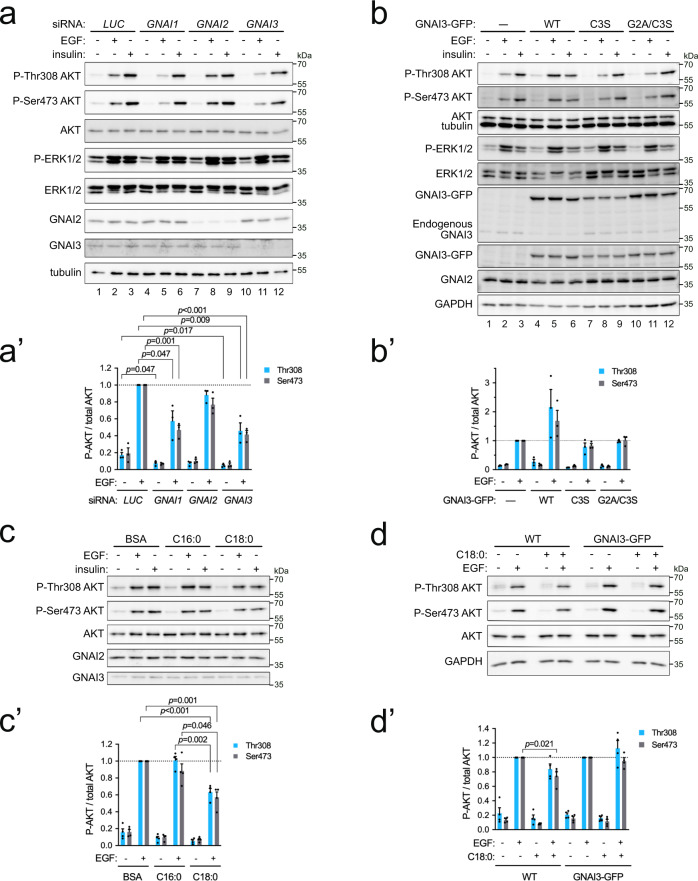
Fig. 6. Exposure of cells to C18:0 reduces AKT activation in response to EGF.
a–a′ GNAI1 and GNAI3 are required for maximal AKT activation in response to EGF. Two days after siRNA transfection, cells were treated with 50 ng/mL EGF or 300 ng/mL insulin for 10 min at 37 °C. Quantified in (a′): Data represent mean ± SEM of three biological replicates, p-values were determined by two-sided t-test with correction for multiple comparisons using the Holm–Sidak method. b–b′ GNAI3 needs to be S-acylated to potentiate EGFR signaling. Overexpression of wild-type GNAI3-GFP but not mutants lacking S-acylation enhances AKT activation in response to EGF. As a control, the parental cell line (—) from which the stable GNAI3-GFP expressing cell lines were derived, is used. Quantified in (b′): mean ± SEM of three biological replicates. c–c′ Exposure of cells to stearic acid suppresses EGF-induced activation of AKT. Cells treated 24 h with 100 µM BSA-conjugated stearate (C18:0) or palmitate (C16:0). Quantified in (c′): mean ± SEM of four biological replicates, p-values determined by two-sided t-test with correction for multiple comparisons using the Holm–Sidak method. d–d′ Stable overexpression of GNAI3-GFP rescues the effect of C18:0 on AKT activation. Cells were incubated with C18:0 (100 µM, 24 h) and then treated with 50 ng/mL EGF for 10 min. As a control, the parental cell line (WT) from which the stable GNAI3-GFP expressing cell line was derived, is used. Quantified in (d′): mean ± SEM of four biological replicates, p-values determined by two-sided t-test with correction for multiple comparisons using the Holm–Sidak method.