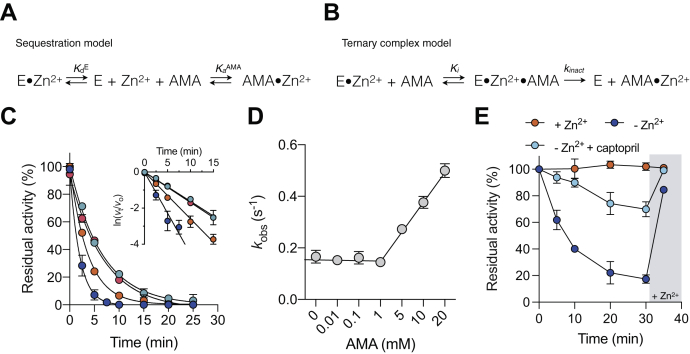
Figure 3.
Kinetic analyses reveal a dual mechanism of NDM-1 inhibition in vitro.A, minimal kinetic scheme for inhibition by sequestration, where E = MBL, KdE = [E][Zn2+]/[E⋅Zn2+], and KaAMA = [AMA⋅Zn2+]/[AMA][Zn2+]. B, minimal kinetic scheme for inhibition by ternary complex formation, where E = MBL, Ki = [E⋅Zn2+][AMA]/[EZn2+⋅AMA] and kinact is the rate constant for the breakdown of the ternary complex to E and AMA⋅Zn2+. C, representative time courses for NDM-1 inhibition by AMA. Rates of inhibition were measured at fixed time intervals with 2 (blue), 5 (orange), 1 (magenta), and 0.1 mM (cyan) AMA. Inset, natural log transformation of the inhibition plot, where vt/vo = residual activity. D, observed rate constants (kobs) determined from the previous panel relative to AMA concentration. E, spontaneous inactivation of NDM-1 in metal-free buffer (blue), metal-free buffer containing l-captopril (100 μM; cyan), and ZnSO4 (10 μM) supplemented buffer (orange). The gray bar denotes the addition of ZnSO4 to each mixture at t = 35 min. Metal-free buffer was produced by treating 25 mM Hepes–NaOH, 1% (v/v) with Chelex-100 resin. All data are representative of the mean value of two independent replicates, and error bars indicate SD. AMA, aspergillomarasmine A; MBL, metallo-β-lactamase; NDM-1, New Delhi MBL-1.