Letter to the Editor:
Inborn errors of immunity (IEI) are monogenic disorders resulting from mutations in genes involved in immune host defense and immunoregulation. Among IEI, CD3 chain deficiencies form a heterogeneous group of autosomal recessive immunodeficiencies and are responsible for a small proportion of T-B + NK+ severe combined immunodeficiency (SCID) [1]. They are characterized by abnormalities of T cell development and function and manifest as early-onset life-threatening infections.
T cell receptor (TCR)/CD3 complex is crucial for antigen recognition. T cells recognize antigen through a heterodimer composed either of the α/β or the γ/δ chains of the TCR [2]. The TCR heterodimer is associated with the γ, δ, ε, and ζ subunits of the CD3 complex that converts ligand recognition by the TCR into intracellular signals. However, the relative contribution of each CD3 protein to the mechanism of signal propagation and its consequences on T cell ontogenesis and function remains only partially defined.
While human CD3 δ, ε, and ζ deficiency lead to SCID, all the previously reported human cases of CD3γ deficiency (14 subjects described in the literature) manifest with variable degrees of combined immune deficiency (CID). It has not been defined yet whether the less severe phenotype of human CD3γ deficiency is due to residual protein expression associated with hypomorphic mutations or to a partially redundant role of CD3γ protein within the TCR/CDR3 complex.
Here, we report in detail a child with biallelic CD3G genetic variant and a clinical phenotype consistent with CID with autoimmunity (P1 in ref. 8). We show for the first time that the variant leads to complete absence of protein expression while preserving polyclonal CD3+ T cell ontogenesis and less severe phenotype.
The patient was adopted at 2 months of age and no family history is available. At 4 months of age, she developed persistent wheezing poorly responsive to bronchodilators. At 6 and 9 months of age, she had two chest X-ray–confirmed pneumonias treated in the hospital with IV antibiotics and O2 supplementation. At 12 months of age after arrival in the USA, upon informed consent, the infant was enrolled in protocol 0409113R approved by the Children’s Hospital Boston IRB. Immunological evaluations revealed a combined immunodeficiency with normal absolute CD3+ T cell numbers, CD4+ T cell lymphopenia (627 cells/μl) with a reduced proportion of naïve T cells and T regulatory cells, increased frequency of memory CD4+, CD8+ cells, and CD45RA+CCR7− CD8+ T cells (TEMRA). High-throughput sequencing of TCR Vβ (TRB) repertoire in sorted CD4+ T cells, CD8+ T cells, and T regulatory cells in the patient demonstrated a polyclonal repertoire with reduced diversity (Supplementary Fig. 1a) and prominent clonotypic expansions (Supplementary Fig. 1b), associated with a higher proportion of unique clonotypes containing cysteines at the apex of the CDR3 (Supplementary Fig. 1c), consistent with a T cell repertoire enriched for self-reactive specificities likely due to defective thymic selection [3].
Reduced numbers of switched and unswitched memory B cells, hypogammaglobulinemia (IgG 338 mg/dL), and impaired vaccination response to Haemophilus influenzae type B and Pneumococcus were also present (Table 1). The child was started on immunoglobulin replacement and antimicrobial therapy, but she continued to have frequent respiratory tract infections over the first year of life. At 14 months of age, she was hospitalized for bilateral pneumonia that required intravenous antibiotics and O2 supplementation for 2 weeks. All blood and respiratory cultures were negative. One month later, she had a second hospitalization for persistent watery diarrhea. Despite treatment with metronidazole for C. difficile toxin positivity by PCR and repeatedly negative stool cultures, the patient continued to suffer from progressively worsening GI manifestations for the first 2 years of life with consequent failure to thrive (weight below third percentile) and TPN requirement at 22 months of age for 3 months. At 2 years of age, she developed severe autoimmune hemolytic anemia (AIHA) (Hb 6.2 g/dL). The persistence of severe recurrent respiratory infections, gastrointestinal symptoms, and AIHA prompted additional studies. EBV viremia (102,000 copies/mL) was documented. Bronchoalveolar lavage (BAL) showed neutrophilia and led to isolation of Klebsiella pneumoniae. Lung biopsy was significant for interstitial lymphoplasmacytic infiltrate with positive in situ hybridization for Epstein-Barr encoding region (EBER). Upper and lower endoscopy showed chronic active gastritis and colitis, and biopsies of small intestine and colon were consistent with autoimmune enteropathy. Linear staining along the periapical border of the enterocytes was positive for anti-enterocyte auto-antibodies. The patient was treated with antibiotics for Klebsiella infection. Rituximab, Sirolimus, and steroids were initiated for the treatment of AIHA, chronic interstitial lung disease, enteropathy, and EBV viremia. The patient responded to the immunosuppressant therapy with significant improvement of the diarrhea (weight: 20th percentile), of the AIHA (Hb11.1 g/dL), and partial improvement of the chronic lung disease by chest CT up to transplant. EBV viral load on blood was undetectable before transplant. When she was 32 months old, the patient received a graft from an 8/10 human leukocyte antigen (HLA)-B and -C matched unrelated donor that was CMV and EBV positive. She received a reduced intensity conditioning regimen with fludarabine 150 mg/m2, alemtuzumab 0.6 mg/kg (total dose, from day 8 to day 6), and busulfan 3.2 mg/kg/day (from day 6 through day 3). Despite receiving GVHD prophylaxis with tacrolimus and mycophenolate mofetil (600 mg/m2/dose q12hrs through day 100), the patient developed stage II, grade 2 acute GI-GVHD that eventually responded to steroid. Three months post-transplant, she was full myeloid donor chimerism; however, she suffered from multiple post-HCT complications including EBV-associated smooth muscle tumor in the lungs, pulmonary aspergillosis, recurrent MRSA bacteremia, and CMV reactivation. She ultimately died ~18 months after the procedure due to CMV reactivation and acute respiratory failure from pneumonitis.
Table 1.
Clinical and immunological phenotype of CD3G-mutated patient
| At diagnosis (12 months of age) | 15 months of age | 2 years of age (onset of AIHA) | 2.5 years of age (pre-BMT) | |
|---|---|---|---|---|
| Hemoglobin (g/dL) | 13.2 (10.5–15) | 10.3 (10.5–15) | 6.2 (10.9–15) | 11.5 (11.5–15) |
| PLT (cells × 103/μL) | 420 (150–450) | 365 (150–450) | 315 (150–450) | 365 (150–450) |
| WBC (cells × 103/μL) | 12.4 (7.4–14.3) | 10.3 (7.4–14.3) | 4.76 (7.4–14.3) | 6.39 (5–15.5) |
| ANC (cells × 103/μL) | 5.8 (1.5–8.5) | 4.6 (1.5–8.5) | 1.42 (1.5–8.5) | 5.21 (1.5–8.5) |
| ALC (cells × 103/μL) | 5.5 (4–10.5) | 4.7 (4–10.5) | 2.84 (3–9.5) | 0.64 (3–9.5) |
| CD3, cells/μL | 2018 (2100–6200) | 2380 (2100–6200) | 1028 (2100–6200) | 523 (1400–3700) |
| CD4, cells/μL of which are: | 627 (1300–3400) | 723 (1300–3400) | 517 (1300–3400) | 243 (700–2200) |
| naïve, % | 23.9 (46–84) | NA | 13.4 (46–84) | NA |
| CM, % | 59 (13–48) | 63.7 (13–48) | ||
| EM, % | 16.8 (0.9–6) | 21.5 (0.9–6) | ||
| EMRA, % | 0.3 (0–1.3) | 1.3 (0–1.3) | ||
| CD8, cells/μL of which are: | 1139 (620–2000) | 1291 (620–2000) | 437 (620–2000) | 245 (490–1300) |
| naïve, % | 4.1 (38–86) | NA | 5.1 (38–86) | NA |
| CM, % | 1 (6–34) | 8.3 (6–34) | ||
| EM, % | 26.8 (0.6–12) | 37.3 (0.6–12) | ||
| EMRA, % | 68.1 (0.5–24) | 49.3 (0.5–24) | ||
| Tregs (CD4+FOXP3+)% | NA | 5.2 (6–13 of CD4+) | 2.5 (6–13 of CD4+) | NA |
| CD19, cells/μL | 1988 (871–1553) | 3054 (871–1553) | 1452 (686–1732) | 52 (686–1732) |
| IgD− CD27+ | 0.3% (1.4–4.1) | NA | NA | NA |
| IgD+ CD27+ | 2.8% (4.2—6.9) | NA | NA | NA |
| CD16/56, cells/μL | 945 (160–950) | 956 (180–920) | 197 (180–920) | 116 (130–720) |
| IgG, mg/dL | 338 pre-IVIG / 691 post-IVIG (400–1300) | NA (IVIG) | NA (IVIG) | NA (IVIG) |
| IgA, mg/dL | < 7 (14–122) | NA | NA | NA |
| IgM, mg/dL | 41(46–160) | NA | NA | NA |
| IgE, kU/L | < 1 (< 200) | NA | NA | NA |
| TRECs (copies of TRECs/μL of DNA) | 125 (> 25) | NA | NA | NA |
| Proliferation to mitogens (PHA, Con-A, anti-CD3) | Decreased | Decreased | NA | NA |
| Infections | RRTI, 2 clinically diagnosed pneumonias within first year of life | RRTI, Clostridium difficile | RRTI, Klebsiella pneumoniae, severe EBV | RRTI, Klebsiella pneumoniae, severe EBV |
| Autoimmunity | Autoimmune enteropathy | Autoimmune enteropathy | Autoimmune enteropathy, GLILD, AIHA | Autoimmune enteropathy, GLILD, AIHA |
Reference values for age are shown in parentheses.
AIHA, autoimmune hemolytic anemia; ALC, absolute lymphocyte count; ANC, absolute neutrophil count; CM, central memory (CD45RA− CCR7+); EBV, Epstein-Barr virus; EM, effector memory (CD45RA− CCR7−); EMRA, effector memory CD45RA+ (CD45RA+ CCR7−); GLILD, granulomatous lymphocytic interstitial lung disease; IVIG, intravenous immunoglobulin treatment; NA, not available; RRTI, recurrent respiratory tract infections
The complex phenotype of infection susceptibility and autoimmunity in the patient led to genetic testing for CID via a 46-gene panel by massively parallel sequencing (BCM-NGS) that revealed a biallelic mutation in the CD3G gene (c. 1A > G) disrupting the translation initiation site (Fig. 1a). The homozygous mutation was confirmed by Sanger sequence analysis (Fig. 1b). We sought to establish whether there was any residual CD3G transcript given that a mutated initiation codon affects ribosome binding and that in vitro data suggest that proper ribosome binding contributes to mRNA stability and protects mRNA from degradation [4]. T cell blasts were generated from the patient and a healthy control by stimulating peripheral blood mononuclear cells with phytohemagglutinin. The CD3G mRNA was amplified with 5-RLM RACE kit (Thermo Fisher Carlsbad, CA), to generate cDNA, and then amplified using a universal forward primer (GGCCACGC GTCGACTAGTAC) and two CD3G-specific 3′ primers designed according to the manufacturer’s instruction (outer primer GGGTGAGGGAAGTATCTTAC encompassing 3′ end of CD3G cDNA, inner primer GCTCACCAGAACAG CAAATA, 150 bp upstream of the outer primer). The product from the 2-step PCR product was analyzed on a standard 2% TAE gel. CD3G cDNA of the expected ~600 bp was readily identified in healthy control T cell blasts (Fig. 1c). However, in the cDNA derived from the patient’s T cell blasts, no CD3G cDNA was identified, indicating that there is complete loss of mRNA transcripts (Fig. 1c) possibly due to mRNA instability and degradation that may be favored by impaired ribosomal binding to the mutated initiation codon [4].
Fig. 1.
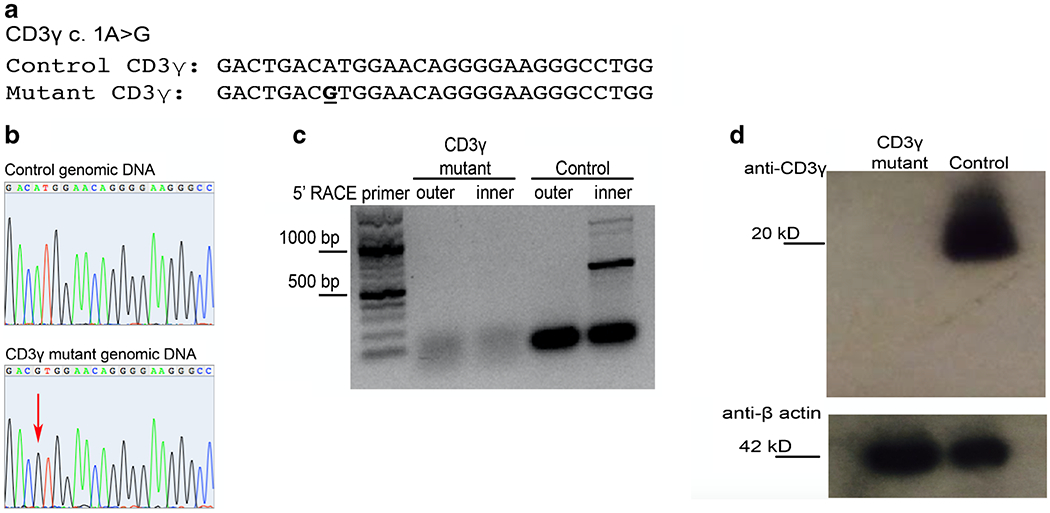
Analysis of CD3G genomic DNA, mRNA, and protein expression in the patient. a Predicted mutation location in exon 1, resulting in disruption of the start codon. b Sanger sequence analysis of exon 1 genomic DNA in control (top) or CD3γ patient (bottom). c 5’-RACE amplification of mRNA isolated from CD3γ-deficient patient or control T PHA lymphoblasts. d Western blot analysis of CD3γ (top) and B-actin loading control (bottom) of protein obtained from lysates of CD3γ-deficient patient or control derived T PHA lymphoblasts
For CD3γ protein analysis, T cell blasts (1 × 106 cells) were utilized to obtain protein lysate. Western blot was performed using the C-terminus CD3γ monoclonal antibody (Abcam Cambridge, UK) and detected using anti-rabbit-HRP (GE Healthcare Marlborough, MA). For loading control, the membrane was stripped and then stained with a monoclonal antibody against beta-actin (Cell Signaling Technology Boston, MA). Western blot analysis of CD3γ demonstrated complete loss of CD3γ protein expression in the patient as compared to the healthy control (Fig. 1d).
Together, these results demonstrate biallelic CD3G c.1 A > G mutation results in the complete loss of CD3G mRNA transcripts and CD3γ protein translation. Recio et al. described two siblings with CD3γ deficiency due to homozygous variant predicted to lead to absent protein expression (c.205A > T K69X) [5]. The subjects’ clinical phenotype was similar to what observed in our patient (severe infections during the first year of life, reduced number of naïve T cells, low IgG, severe autoimmune enteropathy); however, the expression of CD3G mRNA transcript and protein were not investigated. Therefore, our patient is the first definitive example of human CD3γ knockout.
Human studies and murine models have demonstrated that CD3D, CD3E, and CD3Z gene defects lead to severe α/β T cell lymphopenia and SCID [6]. Similarly, Cd3g−/− mice show complete loss of T cell development and SCID [7]. In contrast, CD3γ-deficient patients retain the development of polyclonal T cells (Supplementary Fig. 1) with partially functional α/β TCR/CD3 complex. Furthermore, all of the reported patients with biallelic mutations in the CD3G gene display variable degrees of CID, often associated with autoimmunity [7].
While the data presented here demonstrate that the less severe phenotype observed in patients with CD3γ deficiency (as compared to defects of CD3δ, ε, or z subunits) is not due to residual expression of the CD3γ protein, recent data from our group suggest a model where human CD3γ deficiency alters but does not abrogate surface expression of CD3 and TCR molecules, reducing TCR signaling strength and thereby affecting T cell development, thymus selection, and fate [8].
Supplementary Material
Acknowledgments
We thank the patient and his parents, and healthy volunteers, for participating in this work.
Funding
This study was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, and the Department of Pathology and Laboratory Medicine, Nationwide Children’s Hospital, Columbus, OH.
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s10875-020-00918-z.
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.Tangye SG, Al-Herz W, Bousfiha A, Chatila T, Cunningham-Rundles C, Etzioni A, et al. Human inborn errors of immunity: 2019 update on the classification from the International Union of Immunological Societies Expert Committee. J Clin Immunol. 2020;40(1)24–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alarcón B, Gil D, Delgado P, Schamel WW. Initiation of TCR signaling: regulation within CD3 dimers. Immunol Rev. 2003;191:38–46. [DOI] [PubMed] [Google Scholar]
- 3.Daley SR, Koay HF, Dobbs K, Bosticardo M, Wirasinha RC, Pala F, et al. Cysteine and hydrophobic residues in CDR3 serve as distinct T-cell self-reactivity indices. J Allergy Clin Immunol. 2019; 144(1): 333–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Donnell SM, Janssen SR. The initiation codon affects ribosome binding and translational efficiency in Escherichia coli of cI mRNA with or without the 5′ untranslated leader. J Bacteriol. 2001; 183(4): 1277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Recio MJ, Moreno-Pelayo MA, Kiliç SS, Guardo AC, Sanal O, Allende LM, et al. Differential biological role of CD3 chains revealed by human immunodeficiencies. J Immunol. 2007; 178(4): 2556–64. [DOI] [PubMed] [Google Scholar]
- 6.Fischer A, de Saint Basile G, Le Deist F. CD3 deficiencies. Curr Opin Allergy Clin Immunol. 2005;5(6):491–5. [DOI] [PubMed] [Google Scholar]
- 7.Pacheco-Castro A, Alvarez-Zapata D, Serrano-Torres P, Regueiro JR. Signaling through a CD3 gamma-deficient TCR/CD3 complex in immortalized mature CD4+ and CD8+ T lymphocytes. J Immunol. 1998; 161 (6):3152–60. [PubMed] [Google Scholar]
- 8.Rowe JH, Delmonte OM, Keles S, Stadinski BD, Dobbs AK, Henderson LA, et al. Patients with CD3G mutations reveal a role for human CD3γ in T(reg) diversity and suppressive function. Blood 2018;131(21):2335–44. 10.1182/blood-2018-02-835561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


