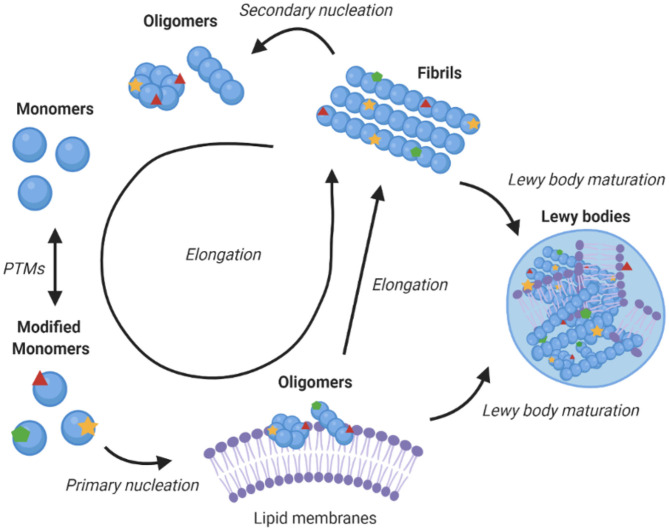
Figure 2.
Role of lipid membranes in α-synuclein aggregation and its links with Parkinson's disease. The figure shows a schematic illustration of a kinetic network model for the aggregation of α-synuclein into amyloid fibrils. Initially, α-synuclein monomers combine to form oligomers, in a heterogeneous nucleation process promoted by lipid membranes (43). These oligomers can then either redissolve or mature and elongate into highly structured amyloid fibrils (44). Once fibrils have been formed, secondary processes, including fragmentation and secondary nucleation, accelerate the aggregation process (45). Downstream to fibril formation, the mechanism of Lewy body formation and maturation remains to be established, although it is likely to involve interaction of different α-synuclein species with lipid membranes (21, 22, 46). The post-translational modifications of α-synuclein can affect essentially all the steps in the aggregation process, including by modulating the binding of α-synuclein to lipid membranes, which may increase the local concentration of α-synuclein and facilitate the initial nucleation events, compared to the slow nucleation rate of free monomers in solution (47).