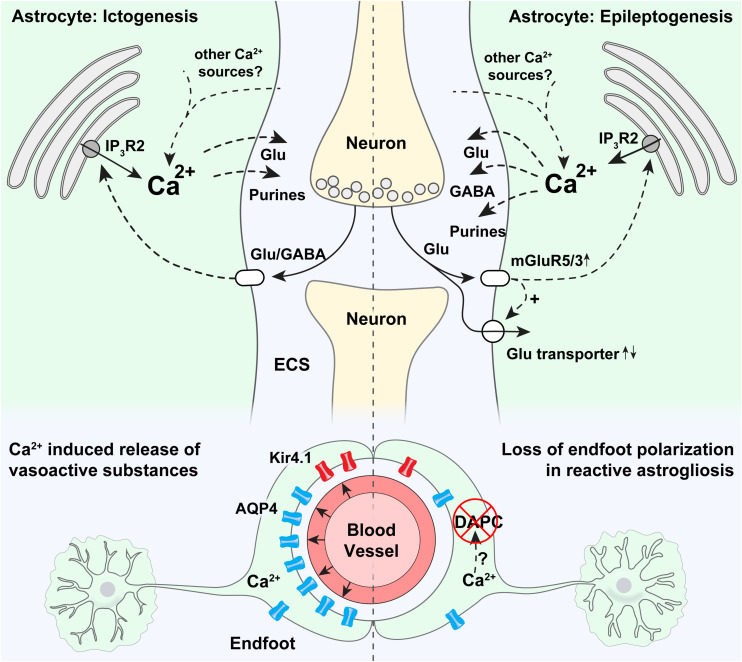
FIGURE 1.
Potential roles of astrocytic Ca2+ signaling in epilepsy. Strong astrocytic Ca2+ signals have been shown to occur in the emergency of acute seizures (in ictogenesis), that are probably triggered by neurotransmitters released by neurons. Ca2+ increases at the onset of seizures are known to be partly mediated by release through IP3R2 from the endoplasmic reticulum, even though pronounced Ca2+ signaling is present also in mice devoid of IP3R2. It is thought that intracellular Ca2+ increases may trigger proconvulsive gliotransmitter release. In astrocytic endfeet, increased Ca2+ signaling has been shown to correlate with ictal vasodilation. Epileptogenesis triggers a pronounced increase in mGluR5 expression, mGluR5-mediated Ca2+ signaling, and increased glutamate uptake. An increase in astrocytic Ca2+ signaling has been demonstrated in the days after status epilepticus, and aberrant Ca2+ signaling at later time points in the epileptogenesis has been anecdotally reported. Increased Ca2+ signaling could potentially cause both the release of glutamate (pro-convulsive), purines (pro-convulsive), and GABA (anti-convulsive, through Bestrophin-1 channels). In astrocytic endfeet in epileptic tissue a pronounced loss of aquaporin-4 (AQP4) and the K+ inwardly rectifying channel Kir4.1 can potentially be due to Ca2+ activated proteases causing a disassembly of the dystrophin associated protein complex (DAPC) tethering AQP4 and Kir4.1 to perivascular endfeet.