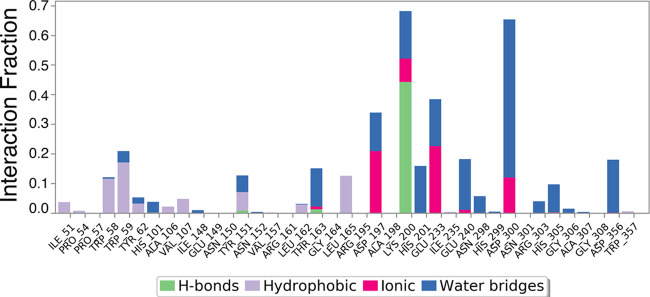
Figure 11.
Bar diagram of α-amylase protein contacts with hexadecanoic acid showing the specific AAs involved in fluctuations and interactions. The X-axis indicates the AA residue number of the α-amylase protein. Protein interactions with the ligand were monitored throughout the simulation. Interactions were categorized by type and summarized in the plot above. α-Amylase protein–hexadecanoic acid interactions (or “contacts”) were categorized into four types: hydrogen bonds, hydrophobic interactions, ionic interactions, and water bridges. The stacked bar charts were normalized over the course of the trajectory: for example, a value of 0.7 suggests that the specific interaction was observed during 70% of the simulation time. Values over 1.0 are possible as some protein residue may make multiple contacts of the same subtype with the ligand.