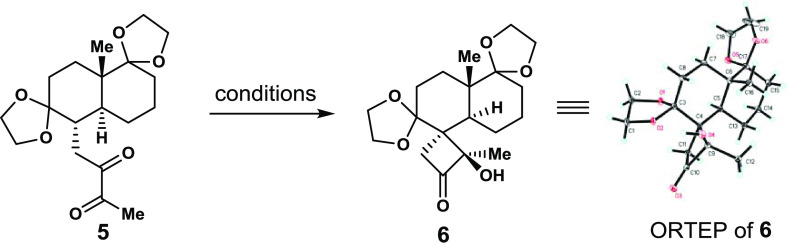
Table 1. Norrish–Yang Cyclization of Diketone 5.
| entry | light source | T (°C) | solvent (0.05 M) | t (h) | yield (%)a | dr |
|---|---|---|---|---|---|---|
| 1 | daylight (65 W) | 30 | MeCN | 8 | 90 | >20:1 |
| 2 | daylight (65 W) | 30 | toluene | 10 | 80 | >20:1 |
| 3 | daylight (65 W) | 30 | CHCl3 | 10 | 96 | >20:1 |
| 4 | daylight (65 W) | 30 | CH2Cl2 | 10 | 84 | >20:1 |
| 5 | daylight (65 W) | 30 | benzene | 10 | 90 | >20:1 |
| 6b | daylight (65 W) | 30 | MeOH | 10 | 68 | >1.5:1 |
| 7 | blue LED (18 W) | 35 | MeCN | 3 | 85 | >20:1 |
| 8 | blue LED (18 W) | 35 | CHCl3 | 3 | 76 | >20:1 |
| 9 | blue LED (18 W) | 35 | CDCl3 | 5 | 77 | >20:1 |
| 10c | blue LED (18 W) | 35 | CHCl3 | 3 | 80 | >20:1 |
| 11d | blue LED (18 W) | 35 | MeCN | 3 | 85 | >20:1 |
| 12d | blue LED (18 W) | 35 | MeCN | 3 | 85 | >20:1 |
| 13 | none | 35 | CHCl3 | 12 | 0 | |
| 14e | sunlight | 37 | CHCl3 | 12 | 64 | >20:1 |
| 15f | daylight (65 W) | 30 | CHCl3 | 12 |
Reagent and conditions: the flask for the photocyclization of 5 (0.05 mmol) dissolved in a solvent was irradiated under the light source listed in the table, and the distance between the flask and the light source was ca. 4–5 cm. Product 6 was purified by flash chromatography on silica gel.
6 was obtained as a 1.5:1 mixture of diastereomers.
0.2 equiv of xanthone was added.
0.2 equiv of benzophenone was added.
6 was obtained in ca. 20% with removal of its ketal group.
3.0 equiv of (2,2,6,6-tetramethylpiperidin-1-yl)oxidanyl (TEMPO) was added.