Figure 3.
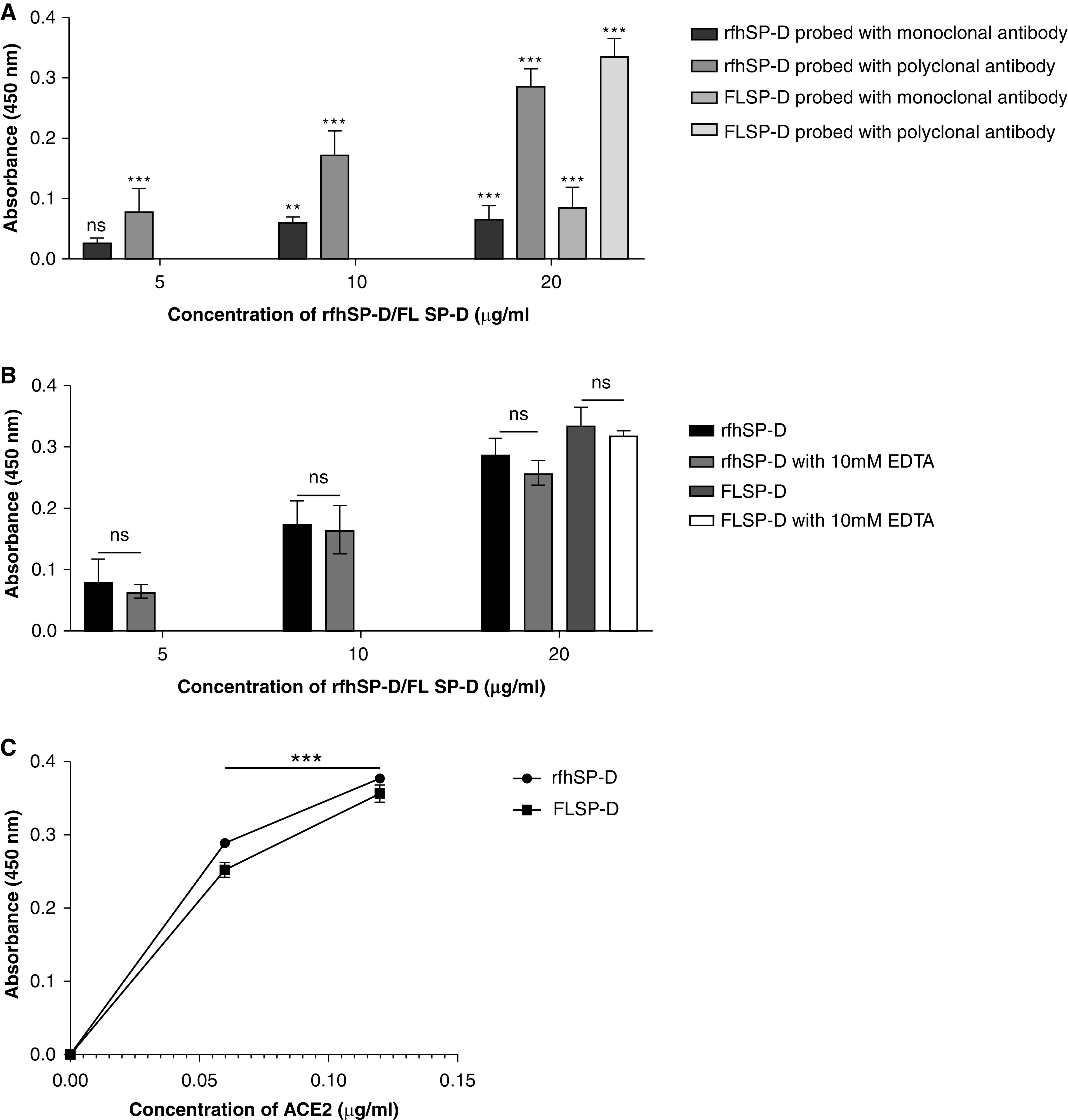
rfhSP-D binds to the immobilized S protein of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2); immobilized rfhSP-D binds to human ACE-2 (hACE-2) in a dose-dependent, but calcium-independent, manner. ELISA showing binding of rfhSP-D to the immobilized S protein. Microtiter wells were coated with 0.3 μg/ml (0.54 nM) of S protein. rfhSP-D (20, 10, and 5 μg/ml or 0.334, 0.167, and 0.083 μM in PBS with 5 mM CaCl2) were added to the wells. Full-length (FL) SP-D (20 μg/ml or 0.038 μM) was also used in a similar manner. BSA (20 μg/ml) was used as a non-specific protein control (mean of the normalized triplicates ± SEM = 0.07 ± 0.006 with polyclonal antibody and 0.025 ± 0.006 with monoclonal antibody). (A) S protein–SP-D binding was detected with either polyclonal or monoclonal antibodies against SP-D. (B) To assess the effect of calcium in the SP-D–S protein interaction, rfhSP-D (20, 10, and 5 μg/ml or 0.334, 0.167, and 0.083 μM) and FL SP-D (20 μg/ml or 0.038 μM), either with or without 10 mM EDTA, was used in a similar manner and probed with polyclonal antibodies against SP-D. (C) The binding of immobilized rfhSP-D to hACE-2 was assessed by coating microtiter wells with 0.1 μg/ml of FL SP-D (1.9 nM) or rfhSP-D (16.7 nM). BSA (0.1 μg/ml) was used as a non-specific protein control (mean of the normalized triplicates ± SEM = 0.006 ± 0.005). Decreasing concentration of hACE-2 (0.12, 0.06, and 0.00 μg/ml or 0.52, 0.26, and 0.0 nM) was added to the wells. The SP-D–hACE-2 binding was detected using streptavidin-HRP. The background was subtracted from all data points. The data were expressed as the mean of triplicates ± SD. Significance was determined using the two-way ANOVA (n = 3) test. **P < 0.05 and ***P < 0.0001. ns = no significance.
