Abstract
This scientific commentary refers to ‘Systemic infection exacerbates cerebrovascular dysfunction in Alzheimer’s disease’ by Asby et al. (doi:10.1093/brain/awab094).
This scientific commentary refers to ‘Systemic infection exacerbates cerebrovascular dysfunction in Alzheimer’s disease’ by Asby et al. (doi:10.1093/brain/awab094).
Cerebrovascular dysfunction is commonly associated with Alzheimer’s disease and can also lead to dementia on its own (vascular dementia). Cerebrovascular dysfunction in the ageing brain and in dementia is associated with breakdown of the blood–brain barrier followed by leakage of neurotoxic blood-derived products into the brain, cerebral blood flow reductions and dysregulation, and often reduced vascular density (reviewed in Sweeney et al.1). Clinically, blood–brain barrier breakdown can be detected using dynamic contrast enhanced MRI (DCE-MRI) with gadolinium-based contrast agents, and/or T2* and susceptibility weighted imaging (SWI)-MRI sequences for detection of microbleeds. It can also be identified through the use of CSF biomarkers of blood–brain barrier breakdown such as increased albumin CSF/plasma quotient, CSF fibrinogen, and CSF plasminogen, as well as increased CSF levels of soluble platelet-derived growth factor receptor beta (PDGFRβ), a marker of blood–brain barrier-associated pericyte cell injury.1-4
In human post-mortem studies, vascular pathology is found in a majority of Alzheimer’s disease cases.5 Notably, cerebrovascular dysfunction in Alzheimer’s disease correlates well with cognitive decline and is further exacerbated by Alzheimer’s disease risk genes, such as apolipoprotein E4 (APOE4), the major genetic risk factor for Alzheimer’s disease.4,6 Recent imaging and biomarker studies have shown that cerebrovascular dysfunction, particularly blood–brain barrier breakdown, is an early biomarker of cognitive dysfunction, and can precede and predict cognitive decline.3,4 In this issue of Brain, Asby and colleagues7 examine the effect of systemic infection on inflammatory markers and exacerbation of cerebrovascular dysfunction in patients with Alzheimer’s disease and vascular dementia.
In their study, Asby et al.7 analysed post-mortem brain samples (superior temporal lobe) from patients with Alzheimer’s disease or vascular dementia, and age-matched controls using a combination of multiplex and traditional ELISA assays to measure inflammatory cytokines and markers of cerebrovascular dysfunction. About 50% of the subjects in each group had systemic infection reported as the primary cause of death. As expected, many brain cytokine levels were elevated in Alzheimer’s disease, vascular dementia or by systemic infection. For example, granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin (IL)-5, and IL-13 were elevated in all dementia groups and in controls with systemic infection. IL-15 and IL-17 were elevated in Alzheimer’s disease cases both with and without systemic infection, while IL-1β and IL-6 were elevated only in Alzheimer’s disease cases with systemic infection. In vascular dementia, IL-1β and IL-17 were elevated without systemic infection, but IL-15 only with systemic infection.
Next, the authors used increased vascular endothelial growth factor-A (VEGF-A) and decreased myelin-associated glycoprotein:proteolipid protein-1 (MAG:PLP1) ratio as surrogate markers suggestive of brain hypoperfusion. They showed that the MAG:PLP1 ratio was reduced in all Alzheimer’s disease and vascular dementia groups and in controls with systemic infection, whereas VEGF-A was increased only in Alzheimer’s disease and vascular dementia groups with infection. Fibrinogen content in brain samples, a marker of blood–brain barrier breakdown, was elevated in Alzheimer’s disease and vascular dementia groups regardless of infection status, and in controls with systemic infection. The blood–brain barrier-associated pericyte marker PDGFRβ was reduced in Alzheimer’s disease and vascular dementia groups with and without infection, consistent with pericyte loss and blood–brain barrier breakdown, as previously reported.6 Endothelin 1 (EDN1), which induces vasoconstriction, was only increased in Alzheimer’s disease groups, consistent with previous reports.8 Taken together, the data presented by Asby et al.7 show that while elevations of some inflammatory and cerebrovascular dysfunction markers are common in Alzheimer’s disease, vascular dementia and systemic infection, and even exacerbated by infection in Alzheimer’s disease and vascular dementia cases, some markers are more specific to the particular disease diagnosis.
How did systemic infection and markers of cerebrovascular dysfunction relate to classical Alzheimer’s disease biomarkers? To address this question, the authors next investigated insoluble amyloid-β42 levels, Braak stage, and APOE4 carrier status. Interestingly, amyloid-β42 levels were not affected by systemic infection in any of the groups studied. Significant correlations between amyloid-β42, fibrinogen, and EDN1 levels were found at advanced (Braak stage V–VI) disease stages. Cytokines IL-15 and IL-17 increased from Braak stage 0–II to Braak stage V–VI, while the MAG:PLP1 ratio and PDGFRβ levels decreased, and VEGF-A and fibrinogen increased, indicating increased inflammation and cerebrovascular disruption with Braak stage. Additionally, APOE4 carriers with Alzheimer's disease had significantly more IL-6, IL-13 and fibrinogen in their brains compared to APOE4 non-carriers, indicating that APOE4 can further aggravate certain cytokine responses related to vascular pathophysiology. Intriguingly, the authors identified several cytokines (IFN-γ, IL-2, IL-4, IL-6, IL-10, IL-12p70, IL-13 and TNF-α) that correlated with markers of vascular dysfunction in non-demented (Braak stage 0–II) controls without apparent infection. The authors concluded that hypoperfusion and blood–brain barrier leakage are associated with Alzheimer’s disease and vascular dementia independently of insoluble amyloid-β levels and they worsen with infection and elevated cytokine levels.
The main take-home message of the study is clear: cerebrovascular dysfunction is an inseparable partner of dementia and may be further aggravated by systemic infections. The reduction in brain PDGFRβ levels in all dementia groups with or without infection is indicative of a reduction in the number of pericytes and/or could be associated with shedding of this receptor upon pericyte damage that can be detected in human CSF in the form of soluble PDGFRβ.2,9 Considering the important role of pericytes in the maintenance of blood–brain barrier function, it is not surprising that the deposition of fibrinogen into the brain parenchyma was increased.10 Since fibrinogen is a relatively large molecule (340 kDa), elevated levels of fibrinogen indicate that other toxic molecules such as thrombin, plasmin and/or autoantibodies against different neuronal receptors are also likely to extravasate into the brain parenchyma due to blood–brain barrier breakdown.1
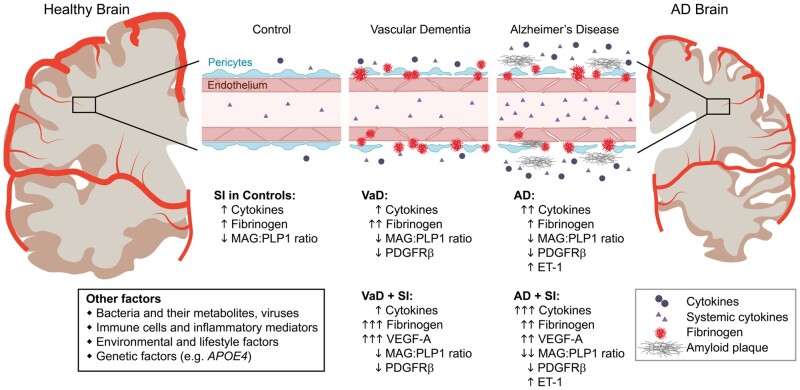
To detect dangerous blood–brain barrier leaks and to relate them to mediators of systemic infection, longitudinal studies that include evaluation of CSF, blood and MRI biomarker data are needed. Another question is whether lower-grade chronic inflammation, environmental and lifestyle-related causes of inflammation such as air pollution or diabetes, or dysbiosis of the gut microbiota, might act through the same pathways as systemic infection-mediated inflammation in dementia (Fig. 1). Future longitudinal studies in living humans are needed to determine whether or not exacerbation of blood–brain barrier dysfunction due to peripheral inflammation further aggravates cognitive decline. Whether developing therapeutics targeting peripheral inflammation and/or elevated cytokines could lead to novel treatments for dementias as well as systemic infections remains an interesting question and topic for future studies.
Figure 1.
Common features of cerebrovascular dysfunction in vascular dementia and Alzheimer’s disease. Both vascular dementia (VaD) and Alzheimer’s disease (AD) share markers of cerebrovascular dysfunction and elevated cytokines that are further exacerbated by systemic infection (SI), as shown by Asby et al.7 Other factors can also influence or aggravate elevation of these markers.
Funding
The work of B.V.Z. is supported by the National Institutes of Health (NIH) grant nos. R01NS034467, R01AG023084, R01AG039452, R01NS100459, R01NS117827, P01AG052350, and P30AG06653, in addition to Cure Alzheimer’s Fund, and the Foundation Leducq Transatlantic Network of Excellence for the Study of Perivascular Spaces in Small Vessel Disease reference no. 16 CVD 05.
Competing interests
The authors report no competing interests.
References
- 1. Sweeney MD, Sagare AP, Zlokovic BV.. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018;14(3):133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sweeney MD, Sagare AP, Pachicano M, et al. A novel sensitive assay for detection of a biomarker of pericyte injury in cerebrospinal fluid. Alzheimers Dement. 2020;16(6):821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nation DA, Sweeney MD, Montagne A, et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25(2):270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Montagne A, Nation DA, Sagare AP, et al. APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature. 2020;581(7806):71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kapasi A, DeCarli C, Schneider JA.. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 2017;134(2):171–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Halliday MR, Rege SV, Ma Q, et al. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J Cereb Blood Flow Metab. 2016;36(1):216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Asby D, Boche D, Allan S, Love S, Miners JS.. Systemic infection exacerbates cerebrovascular dysfunction in Alzheimer’s disease. Brain. 2021;144(6):1869–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nortley R, Korte N, Izquierdo P, et al. Amyloid beta oligomers constrict human capillaries in Alzheimer’s disease via signaling to pericytes. Science. 2019;365(6450):eaav9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sagare AP, Sweeney MD, Makshanoff J, Zlokovic BV.. Shedding of soluble platelet-derived growth factor receptor-beta from human brain pericytes. Neurosci Lett. 2015;607:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nikolakopoulou AM, Montagne A, Kisler K, et al. Pericyte loss leads to circulatory failure and pleiotrophin depletion causing neuron loss. Nat Neurosci. 2019;22(7):1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]