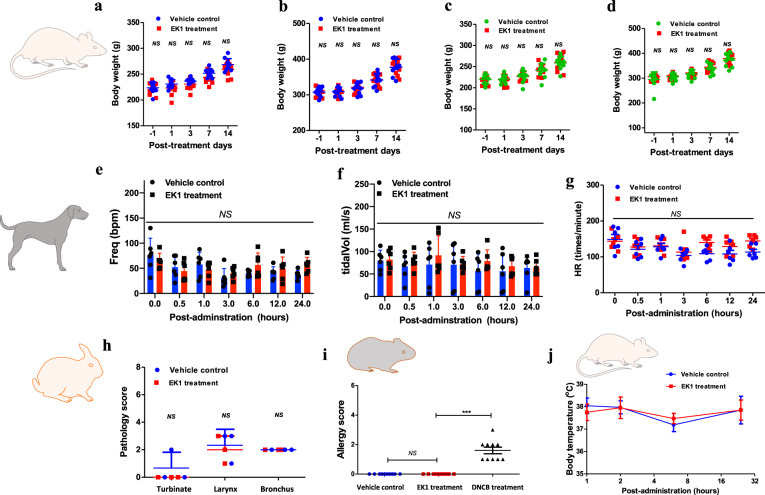
Fig. 6.
Preclinical safety of EK1 peptide in a pharmaceutical formulation. a–d In vivo safety of EK1 peptide on SD rats. The body weight of female (a) and male (b) SD rats treated by EK1 peptide (75 mg/kg) through the aerosol inhalation route. Body weight of female (c) and male (d) SD rats treated with EK1 peptide (180 mg/kg) through the tail vein injection route. e–g Effect of EK1 peptide (24 mg/kg) through the aerosol inhalation route on cardiopulmonary function in beagle dog model, including respiratory frequency (e), tidal volume (f), heart rate, HR (g). Those indicators were monitored by the emkaPACK4G animal physiological signal telemetry system. h “Nasal mucosa irritation test” of EK1 peptide (0.88 mg/kg) on a rabbit model. Pathogenic score is based on epithelial cell shedding, goblet cell hyperplasia, eosinophilic inflammatory cell invasion and mixed inflammatory cell invasion. i “Active skin allergy test” of EK1 peptide (4.0 mg/kg) on guinea pig model. j Effect of EK1 peptide (18 mg/kg) on the central nervous system. After administration of EK1 peptide through aerosol inhalation, the body temperature changes on SD rat model. ***P < 0.001; “NS” indicates no statistical difference