Abstract
Background
Since 2020 SARS-CoV-2 spreads pandemically, infecting more than 119 million people, causing >2·6 million fatalities. Symptoms of SARS-CoV-2 infection vary greatly, ranging from asymptomatic to fatal. Different populations react differently to the disease, making it very hard to track the spread of the infection in a population. Measuring specific anti-SARS-CoV-2 antibodies is an important tool to assess the spread of the infection or successful vaccinations. To achieve sufficient sample numbers, alternatives to venous blood sampling are needed not requiring medical personnel or cold-chains. Dried-blood-spots (DBS) on filter-cards have been used for different studies, but not routinely for serology.
Methods
We developed a semi-automated protocol using self-sampled DBS for SARS-CoV-2 serology. It was validated in a cohort of matched DBS and venous-blood samples (n = 1710). Feasibility is demonstrated with two large serosurveys with 10247 company employees and a population cohort of 4465 participants.
Findings
Sensitivity and specificity reached 99·20% and 98·65%, respectively. Providing written instructions and video tutorials, 99·87% (4465/4471) of the unsupervised home sampling DBS cards could be analysed.
Interpretation
DBS-sampling is a valid and highly reliable tool for large scale serosurveys. We demonstrate feasibility and accuracy with a large validation cohort including unsupervised home sampling. This protocol might be of big importance for surveillance in resource-limited settings, providing low-cost highly accurate serology data.
Funding
Provided by Bavarian State Ministry of Science and the Arts, LMU University-Hospital; Helmholtz-Centre-Munich, German Ministry for Education and Research (project01KI20271); University of Bonn; University of Bielefeld; the Medical Biodefense Research Program of Bundeswehr-Medical-Service; Euroimmun, RocheDiagnostics provided discounted kits and machines
Keywords: COVID-19, SARS-CoV-2, Dried blood spot, DBS, Filter paper, Antibody, Serology, Roche Elecsys, Nucleocapsid
Research in context.
Evidence before this study
In spring 2020 it became evident that the SARS-CoV-2 epidemic was a great challenge to track in populations around the world. This was most obvious in settings with limited resources, under developed public health infrastructures and limited laboratory capacities. Therefore, we looked into the use of dried blood spots on filter paper (DBS) for SARS-CoV-2 serology. Database search in medline/pubmed at the commence of the study 31st of March 2020 was performed using DBS/Filter paper/Dried Blood Spot combined with Coronavirus/SARS-CoV-2/Sarscov2/Covid-19 in the respective combinations. There were only seven results (six prior to 2015, not dealing with SARS-CoV-2 and one referring to deep brain stimulation), thus nothing relevant regarding the use of DBS in SARS-CoV-2 serology could be found.
Added value of this study
For comparable DBS sampling for SARS-CoV-2 serology, a reliable, cost effective and accurate protocol with thorough standard operating procedures is needed. This study provides such a protocol based on the Roche Elecsys Anti-SARS-CoV-2 anti-N assay. It also presents data of two studies using this assay, one with more than 10000 participants and one with home sampling of more than 4000 participants. This demonstrates the feasibility of the approach for global health purposes. Although, just very recently a few protocols have been published using a similar approach, non to our knowledge could (i) provide data of a large validation cohort with matching venous blood samples, including large proportions of subjects with low titres, (ii) demonstrate the feasibility in a sufficient unsupervised home sampling cohort, (iii) demonstrate feasibility of the respective protocol for large sample sets by detailing an automated workflow and providing data of larger cohorts.
Implications of all the available evidence
The performance of DBS sampling coupled with automated Roche Elecsys based serology is very good. The approach lends itself to be used as a public health tool especially for resource limited settings and allows the analysis of large amounts of samples in a short time with high precision. Roche Elecsys machines are distributed worldwide and the price per measurement is modest, with a good global coverage of supply chains. The study here demonstrated the approach for anti-N serology, which with S1/RBD based vaccines entering the market, will over the long run have to be complemented with anti-S1 DBS based serology to differentiate vaccination titres from infection titres.
Alt-text: Unlabelled box
1. Introduction
The severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) causing COVID-19 was reported for the first time on 31st December 2019 in the city of Wuhan (Hubei province, China) as a cluster of pneumonia cases of unknown aetiology [1]. The virus rapidly spread all over the world originating into the fifth documented pandemic as declared by the World Health Organization (WHO) after the Spanish flu in 1918–1920 [2]. Despite warnings and early implementation of public health mitigation strategies by the health authorities, the virus continues to spread globally and as per March 2021 there are more than 119 million confirmed cases worldwide with more than 2.6 million fatalities [3]. The ongoing pandemic posed a great challenge for diagnostic capacities even in countries with highly developed health care systems. Since early 2020 the U.S. Food and Drug Administration (FDA) has authorized the emergency use of different in-vitro diagnostic assays (IVD) for the detection of the infection [4]. In the current diagnostic landscape, acute infections are generally diagnosed via real time RT-PCR of fresh respiratory samples. Positivity thereby is often observed only for a few days. Hence, for epidemiological questions such as disease spread or prevalence in the population, serological testing is needed. Serological parameters are generally measured in venous blood. The collection of blood from a large number of individuals is always challenging due to the required capacity, the relatively high cost and the medical personnel needed to obtain the samples [5]. These constraints are especially pronounced in developing countries. The establishment of high throughput serology tests relying on an easy-sampling-strategy is therefore essential for effective serological tracking of the pandemic eventually also allowing the gradual reopening of the economy.
In the past, dried blood spot (DBS) based specimen collection has been extensively used in large scale population screening programs [6,7]. DBS is preferred to the conventional venipuncture as it is less invasive, does not require medical personnel to obtain and after drying is stable for extended times under different conditions. Besides, infectivity of bloodborne pathogens is reduced after drying, thus posing less of a risk for personnel handling the samples. Still, sufficient precautions have to be maintained, as not all handling errors can be excluded, and some blood borne pathogens are less easily inactivated by drying. Recently, the method has been reported to show promising results when used for the home-based sampling of blood for SARS-CoV-2 antibody screening especially in high-risk populations [6,8]ϕ. In our study, we take advantage of the DBS sampling strategy to identify anti-N serological signatures of SARS-CoV-2 infection using the Roche Elecsys Anti-SARS-CoV-2 anti-N assay. The assay has demonstrated sensitive and robust performance characteristics when used with serum or plasma obtained from venous blood samples [9]. Here, we describe the performance of our protocol for high throughput semi-automated DBS-based anti-N serology, and demonstrate its utility for remote sampling with a total of 16687 samples in three independent datasets.
2. Materials and methods
2.1. Patient samples
Participant's samples for establishing and validating the method were obtained within a randomly selected population-based Covid-19 cohort study (KoCo19) in Munich, Germany. The primary results and the selection process were reported elsewhere [10,11]2f. In brief, a longitudinal and representative population based cohort was recruited to follow up the seroprevalence and -incidence in Munich. The first sampling round was performed by venipuncture starting in March/April 2020. Study participants were recruited during the lockdown in their homes by trained study teams using a random walk procedure. Inclusion criteria were the presence of at least one household member ≥ 18 years of age with sufficient command of the German language to give informed consent for participation in the study. Participation then was possible ≥ 14 years of age and permanently living in the respective household. After a certain date, the teams started to additionally collect DBS samples from all participants joining the study. Besides the population based arm, in the KoCo19 group of studies, also COVID-19 infected patients were specifically recruited, obtaining additional DBS samples with matching venous blood samples. This resulted in DBS used for the evaluation of different elution buffers and serological tests (or their prototypes) for the use with DBS. Blood was collected in ethylenediaminetetraacetic acid (EDTA)-coated tubes and maintained at 4 °C until centrifugation to isolate plasma. Plasma samples were stored at –80 °C in temperature-controlled biobank freezers. DBS samples were prepared using single-use safety lancets for capillary puncture (Sarstedt safety 85.1016). Capillary blood from the finger was dropped onto barcode labeled neonatal screening filter cards (8.460.0004.A Rev.1, Ahlstrom-Munksjö) and left to dry for 12–24 h at room temperature protected from direct sunlight. Filter paper cards were stored at -80 °C or 4 °C in plastic boxes until they were used for analysis. Finally, 100 sample pairs (50% positive) were used for the establishment of the protocol, which was then validated using 1710 additional matched samples.
A second cohort of patients consists of 10247 employees from the large German company Deutsche Post DHL group. In this study, the responsible company physicians used safety lancets to obtain DBS on the same filter paper model from employees. 23320 employees were invited to take part in the study using digital posters in the intranet of the company as well as notifications on the company app. About 43.9% showed up at the company physician and agreed to participate. The samples were batched locally and sent to the laboratory by mail in A6 file-card boxes at ambient temperature. These boxes were then sealed in plastic bags with dry air and stored at 4 °C until analysis.
In the third dataset, the participants of the longitudinal KoCo19 study were invited to participate in a follow up of the serological epidemiology study. During October 2020, a total of 5293 blood collection sets (Euroimmun ZV 9701-0101) were sent to the different households. This was accompanied by instructions for use, a link to a video tutorial (https://www.youtube.com/watch?v=vpZUzuQV10E&feature=emb_title) and a participant-specific barcode number to collect their own DBS. A total of 4471 DBS returned to the laboratory (84·5% of the sent sampling kits), 4465 being usable (4 did not have enough material, 2 had labeling mistakes). Upon reception, barcodes were scanned and samples were stored at 4 °C until analysis within the next 48–72 h.
2.2. DBS quality control
Independently of who performed the blood collection, it was requested that at least 2 of the 5 circles on the filter paper card were completely soaked with blood. Instructions were provided to leave the blood droplets to dry at room temperature for 12–24 h in a dry place protected against direct sunlight. Thereafter, the filter cards were packed in air tight zip lock bags or placed in the A6 file-card box with a card between the individual inoculated DBS and shipped by mail to the laboratory. No cross-contamination between DBS cards was observed.
2.3. Extraction of antibodies from the DBS
To achieve a high sample throughput, we established a semi-automated workflow consisting of two Panthera-Puncher™ 9 Instruments (PerkinElmer) which dispense three discs with 3.2 mm diameter per DBS spot into each well of a barcoded 96-well plate respectively. Using two JANUS® G3 workstations (PerkinElmer) with 8-channels pipette-head capable of adjusting the distance between the tips, the elution buffer is dispensed. Elution is performed in temperature-controlled shakers (MIUlab ES-60E) for 1 h at 37 °C with 300 rpm shaking. A total of 80 μl of PBS buffer containing albumin (5%), ammonium thiocyanate (2·5 mM) and Tween 20 (0.5%), is used to elute antibodies off the DBS and suppress background. After elution, well plates are transferred into the second Janus workstation where 60 μl of each eluted blood/buffer solution is transferred to a tray with 15 individually barcoded 5-rack packs of the Roche e801 machine. Roche 13/16 micro sample cups (Roche, 05085713001) are used to keep the volume down needed for analysis in the Roche Elecsys system. In this setup, the sample throughput of the e 801 is the rate limiting factor. Within a 10 h laboratory shift, up to 2500 samples can safely be processed with the workflow described above, excluding repetitions. Alternatively, sample transfer was performed with adjustable tip spacing multichannel pipettes (Integra Voyager) to perform the process manually.
2.4. Roche Elecsys Anti-SARS-CoV-2 immunoassay
The Roche Elecsys Anti-SARS-CoV-2 assay uses a recombinant protein representing the nucleocapsid (N) antigen in a double-antigen sandwich assay format, which favors detection of high affinity antibodies against SARS-CoV-2. This immunoassay was performed by the Cobas e801 analytical unit (Roche), a high throughput immunochemistry module that uses the electrochemiluminescence technology. Roche Elecsys Anti-SARS-CoV-2 is validated for the use on human serum and plasma, providing a positive reaction against SARS-CoV-2 with a cut-off index over 1 (COI≥1). As the elution of the DBS leads to lower concentrations of antibodies in the eluate, a different cut-off was established as explained in more detail in the results section.
3. Statistics
Prior to analysis, we cleaned and locked the data. For the analyses and visualization, we used the software R, version 4.0.2. In each sampling set, only one sample per individual was obtained and included in the statistical analysis; in case of individuals with multiple visits, we only considered the sample with both plasma and DBS (establishment/validation) or just a DBS sample. For operational replicates we used the lowest value in DBS and the last measurement for plasma. Cut-offs were empirically defined minimising the number of false positives/negatives, based on the accordance of matched plasma and DBS eluate results. We derived two sets of cut-offs: (i) a single hard cut-off and (ii) two cut-offs with an intermediate region in between. The intermediate region was primarily designed to identify patients with borderline positivity that could hence be recalled for venous blood sampling. Sensitivity and specificity were calculated for each set of cut-offs referring to the plasma result, giving the percentage of samples positive or negative, respectively, in both DBS and plasma. For association among continuous variables we report square roots R of coefficients of determination.
To compare graphically operational replicates the Bland-Altman plot, also known as difference plot, was applied (Bland & Altman, 1986 and 1999). In this graph the mean of the two measurements is plotted on the x-axes, while on the y-axes the difference between the measurements (or alternatively the ratios) is presented. The thick dashed lines define the mean difference, and the limits of agreement (95% CI) of the mean difference. For these three values the corresponding 95% CI are also plotted, using shorter dashed lines (see supplemental Fig. 1).
4. Data handling
Barcodes of DBS cards were scanned at the puncher. The barcode number is linked automatically with the position on the 96-well plate, which is also labeled and uniquely identifiable with another barcode. With a self-programmed script, data is transferred to the Janus robot, which is loaded with rack packs which are also barcoded. The robot transfers the samples in groups of five into each of the rack packs. In parallel, a worklist for the Cobas analyzer is generated which is transferred to allow automated analysis as well as result export with raw values matched to the respective barcode of the DBS sample.
5. Role of funding source
The funders did not influence the scientific conduct of the experiments or presentation/interpretation of the results or the decision to publish the data.
6. Ethics
The study was conducted in accordance with good clinical (GCP) and epidemiological practice (GEP) standards as well as the Declaration of Helsinki in its most recent form (as amended by the 64th WMA General Assembly, Fortaleza, Brazil, in October 2013). The study protocol was approved by the Institutional Review Board of the Medical Faculty at Ludwig Maximilian University Munich, Germany (opinion dated 31 March 2020; number 20–275), prior to study initiation. Informed consent was obtained from all study participants prior to study inclusion.
7. Results
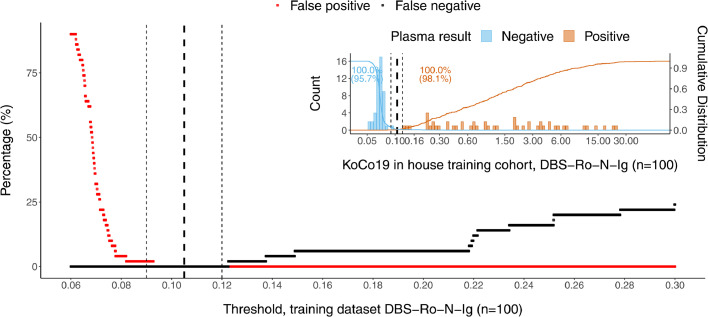
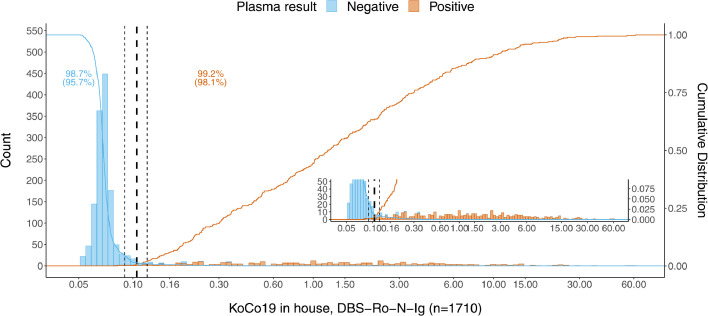
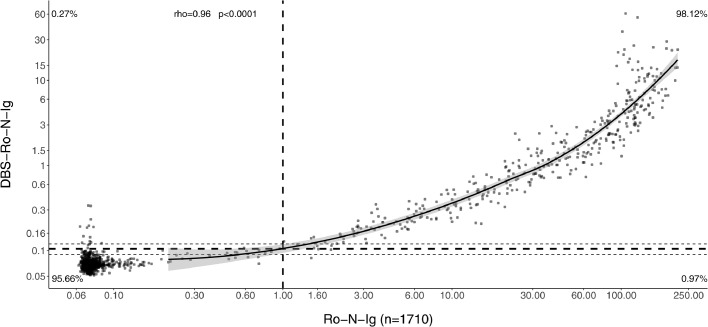
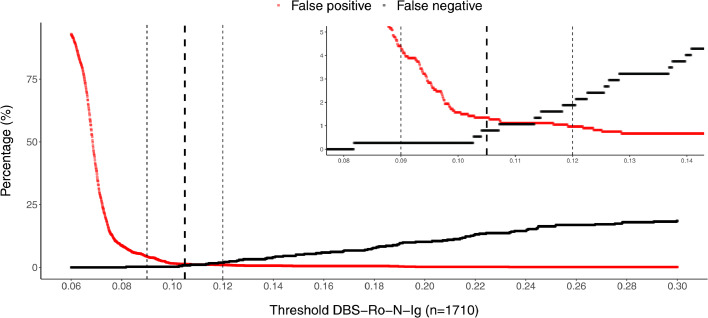
An establishment dataset of 100 DBS/venous sample pairs including 50 positive samples were analyzed by Roche Elecsys Anti-SARS-CoV-2 immunoassay resulting in a distribution of measurements with negative and positive values clearly separated from each other (Fig. 1). Taking into account the technical dead volume of the Cobas e 801 instrument and the Janus pipetting robots, a minimum of 80 µl was required to re-elute the dried blood from a total of three discs. Our elution buffer did not show any increased background compared to plasma. Measuring the DBS eluates, a good separation between positive and negative values was observed (Fig. 1). Cut-off values were determined empirically to optimize sensitivity and specificity, based on the accordance of the plasma and DBS eluate results. With the establishment dataset (n = 100) an estimate of the cut-off of 0.105 was derived. Besides, we established an arbitrary intermediate region around the cut-off (≥0.09–0.12 intermediate, ≥0.12 positive). This was done to compensate for possible variation due to the use of the DBS sampling protocol in unsupervised home sampling by untrained study participants. The cut-off was validated using a larger set (n = 1710) of matched pair samples (Figs. 2, 3, 4). There, we found that 2.98% (51/1710) of patients in the validation dataset were within this intermediate range. Thereby, 45 cases being negative in plasma, 6 positive. Corresponding sensitivity and specificity in the validation were 98.1% and 95·7%, respectively (Figs. 2, 3). Using the single positive/negative cut-off of 0.105, of the 51 intermediate samples only 5 turned out to be false positives and 2 false negatives. Thus, a higher sensitivity and specificity of 99.2% and 98.7% could be obtained. This demonstrates no benefit from introducing an intermediate region. Overall, in 98.77% (1689/1710) of the patients, the assay provided accordant results, with only 0.18% being false negative (3/1710) and 1.05% false positive (18/1710) (Table 1). Samples with raw values above 0.09 [COI] were re-punched from the original material and measured a second time in all studies. To exclude carryover, the two consecutive negative samples following a total of n = 50 positive DBS were compared with the average values of negative samples. No difference could be observed. Finally, the first n = 100 positive samples punched twice were investigated regarding correlation between the first and second value (supplemental Fig. 1), no single value changed the category between positive and negative using our cut-offs, correlation was found to be very high (R = 1.0).
Fig. 1.
Establishment dataset (n = 100) for cut-off estimation plotted as percentages of false positives/negatives depending on a variable threshold for DBS. The insert above right shows the frequency distribution of the detected antibody titre against SARS-CoV-2 in DBS eluates. The dashed vertical lines denote the empirically determined cut-off value (bold) for result classification with its boundary values (light).
Fig. 2.
Frequency distribution of detected antibody titre against SARS-CoV-2 in DBS eluates from patients of our in-house KoCo19 cohort (n = 1710). The dashed vertical lines denote the empirically determined cut-off value (bold) for result classification with its boundary values (light). The insert in the bottom right represents a zoom-in on the y-axis to allow visualization of the lower frequency positive values.
Fig. 3.
Scatterplot illustrating the relationship between antibody titre detected in plasma (x-axis) and the corresponding DBS-eluate (y-axis) (n = 1710). The correlation is calculated using the Spearman method. The dashed vertical line denotes the manufacturer's cut-off value for result classification. The dashed horizontal lines denote the empirically determined cut-off value (bold) for result classification with its boundary values (light). The solid line represents the LOESS (locally estimated scatterplot smoothing or local regression) modelling the association. The grey region is the 95% CI of the LOESS estimate.
Fig. 4.
Empirical cut-off determination (dashed horizontal lines) given as percentages of false positives/negatives depending on a variable threshold for DBS. The empirically determined cut-off value is denoted in bold for result classification with its boundary values (light).
Table 1.
DBS samples compared to matched plasma samples.
| Venous plasma sample | Sensitivity | Specificity | |||
|---|---|---|---|---|---|
| Positive | Negative | ||||
| Experimental Intermediate cut-off ≥ 0·09; Positive cut-off ≥ 0·12 |
Positive | 366 | 13 | 98·1% | 95·7% |
| Intermediate | 6 | 45 | |||
| Negative | 1 | 1279 | |||
| Experimental Positive cut-off ≥ 0·105 |
Positive | 370 | 18 | 99·2% | 98·7% |
| Negative | 3 | 1319 | |||
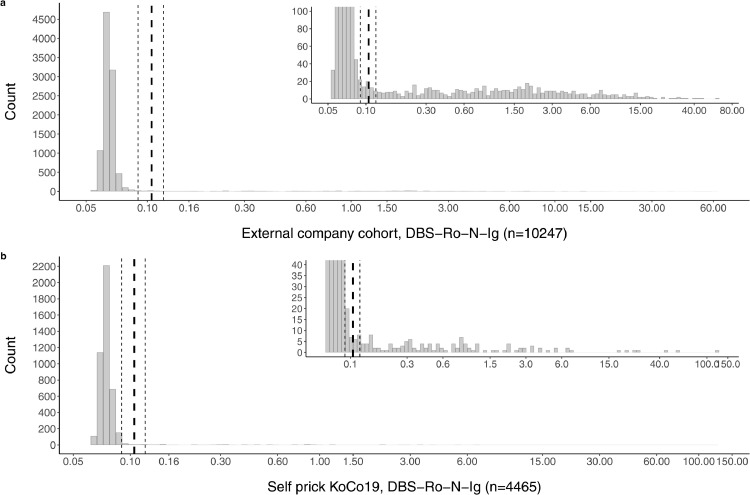
While for the establishment and validation datasets paired samples were available, we performed a second study investigating a total of 10247 external DBS samples of employees from a large company sent to us by mail from their occupational health physicians. With our detailed instructions for sample collection, only 0.04% (4/10247) of DBS cards had to be rejected from processing due to an apparent lack of sample material or equivocal sample labeling. The distribution of the raw values obtained in this large cohort is identical to what was observed in the establishment dataset (Fig. 5a). With this finding, it can be assumed that our protocol is also applicable for large scale seroprevalence studies based on home-sampled DBS.
Fig. 5.
Frequency distribution of detected antibody titre against SARS-CoV-2 in DBS eluates from patients of (a) an external company cohort (n = 10247) and (b) the in-field study cohort (n = 4465). The dashed vertical lines denote the empirically determined cut-off value (bold) for result classification with its boundary values (light). Inserts in the upper right of each sub-figure represent zoom-in on the y-axis to allow visualization of the lower frequency positive values.
In the third dataset 5293 participants from the longitudinal KoCo19 study [10] were invited to prepare self-sampled DBS cards with the aid of a simple Blood Collection Set (Euroimmun ZV 9701-0101) and to send it back for subsequent analysis. From 4471 returned samples 0.13% (6/4471) had to be removed from analysis, not fulfilling the quality standards (2 due to labelling mistakes, 4 due to insufficient materials). In 5 further cases, patients reported to feel uncomfortable to perform the finger pricking and were subsequently offered a venous blood draw at our outpatient facility (not included in the presented dataset). 4465 samples could be analyzed for the in-field cohort. Using the intermediate range approach, we found that only 0.86% (39/4465) of patients were within this region. These patients were invited for a blood draw later on. Only 58.97% (23/39) of them gave blood samples, resulting in n = 17 negatives and n = 6 positive values. However, there were significant delays up to 60 days between DBS and venous sampling, making direct comparisons difficult.
The overall distribution of raw values was found to be identical in the unsupervised home sampling as compared to the other groups. Dropout rates due to insufficient material on the DBS card, or labeling errors were also surprisingly rare (0.13%). Again, the lack of performance gain with the introduction of the intermediate region is apparent (Fig. 5b).
8. Discussion
The main objective of the present study was to investigate DBS as an alternative sampling material for antibody detection by the automated Roche Elecsys Anti-SARS-CoV-2 anti-N immunoassay. The assay was chosen as it shows favorable characteristics, such as high sensitivity as well as specificity and a good separation between the raw values of the positive and negative populations [10,11]3f. It is also fast and the automated workflow allows for high throughput analysis. Further, the assay is attractively priced worldwide and comparable between laboratories. Roche Elecsys infrastructure is also widely distributed globally and should be available at least in centralized laboratories in almost every country in the field of clinical chemistry. Combined with the use of globally widely available neonatal screening cards, this combination also allows for the transfer of the protocol to resource limited settings. Obviously, for protocol automation as described here, larger laboratories are better suited. Still, in many regions of the world, DBS sampling is often the only possible way to obtain large amounts of samples, making such a protocol especially valuable.
To allow for efficient workup of many specimens, an at least semi-automated workflow including punching the DBS as well as liquid handling of the samples is necessary. For this, sufficient volumes are needed to address the minimum volumes for automated robotic pipetting as well as analysis. As the punched paper discs are submerged in buffer solution, they soak and swell to some degree. To extract most of the buffer for analysis, either centrifugation or filtration steps are required, adding complexity and cost to the workflow. We chose to use higher elution volumes (80 µl) to omit these steps. Despite resulting in higher dilutions, the sensitivity and specificity (99.20% and 98.65%, respectively) of the workflow is still excellent even in a large representative sample set with mainly oligo-or asymptomatic patients with predominantly low titres [12].
Another potential source of variation all DBS protocols suffer from, is the haematocrit effect caused by the biological variation of haematocrit values in the human population. Similarly, blood can be diluted with tissue fluid during capillary blood sampling to a varying degree. To account for such effects, we used a large sample set of participants from the representative cohort in Munich as a validation dataset (n = 1710). This group includes a large proportion of subjects with low anti-SARS-CoV-2 titres. The samples used thereby were also not filter cards spiked with blood from venous draws, but real finger prick samples including the inherent increased variability of this sample type. The overall performance of the assay was not impacted significantly by the variabilities described above. The volcano effect as well as coffee stain effect described in DBS samples was in some studies found to influence the concentrations of biomarkers in the DBS spot due to chromatographic or drying effects. We did not observe relevant effects in our samples related to this. All that might be due to the averaging effect achieved by using three individually punched discs per assay. Actually, the position of the punches is automatically selected and spread over the spot, partly mitigating inhomogeneity effects. Further, the test properties of Roche Elecsys Anti-SARS-CoV-2 anti-N with a good raw value separation between positive and negative populations allows for considerable variation of sample concentrations while maintaining a good separation between the positive and negative populations.
By omitting filtration or centrifugation in the process, the risk of fiber fragments from the filter paper distorting the measurement is increased. Such effects might lead to false positive results in rare cases. We decided to repeat all positive measurements to exclude such artifacts as well as other possible mistakes like data transfer errors, handling errors or possible carryover effects. This increased safety leads to the need for a higher sample amount, as well as time-consuming re-punching of the original filter paper. Overall we decided that this effort is worth the increased safety, given the speed of 300 samples per hour. It did also not significantly impair the proportion of samples that could be measured given the overall small amount of sample needed.
We calculated two possible cut-off values, first we derived a cut-off using a set of 100 DBS/venous blood sample pairs (containing 50 positives) which was confirmed in the consecutive 1710 paired finger prick samples. Using one cut-off will provide the overall best performance (sensitivity of 99.20% and specificity of 98.65%). The cut-off values with an indeterminate zone were thought to increase sensitivity and correct for possible handling errors if all subjects testing in this range are later sampled with a venous blood draw. The effect however was found to be low, with only 0.92% (154/16687) being in the intermediate zone. Thus, no significant improvement can be made by venous re-sampling of those subjects as compared to the optimized cut-off.
This protocol uses anti-N antibodies, detecting infections with the wild type SARS-CoV-2 virus. Very recently, two manuscripts provided evidence from small sample sets about the general possibility to use the Roche Elecsys Anti-SARS-CoV-2 anti-N immunoassay from DBS eluates [6,13]. Other targets like Spike protein (S) or Receptor binding domain (RBD) are not assessed. This is a limitation of the protocol as it does not allow for the detection of vaccination titres, or the very rare, but possible anti-N non-responders after natural infection. For epidemiological purposes including vaccination efficacy trials, a DBS based quantifiable or semi-quantifiable anti-S titre measurement would be a desirable addition to the protocol and is currently under investigation in our laboratory.
Contributors
MH, JB and AW designed the study, JB, IP, ZNK and RRA performed laboratory analysis, NC, AB, IK, JO, AT and AW analyzed, verified and visualized the underlying data, JB, RRA, NC, AB, ZNK and AW drafted the manuscript. All authors approved the final version of the manuscript.
Data sharing statement
Raw data not containing any identifiable information will be made available upon qualified request to other research groups.
Declaration of Competing Interest
The study was supported by multiple funders which includes Roche Diagnostics, providing machinery and kits for discounted rates. AW and MH have received personal (advisory) and material support from Roche, which was not related to the conduct of DBS serology presented within this study. The authors have no other relevant conflicts of interest to declare regarding the conduct of the study.
Acknowledgments
This work was conducted within the general umbrella of the KoCo19 representative SARS-CoV-2 cohort in Munich. The whole project was supported by the Bavarian State Ministry of Science and the Arts, the University Hospital of the LMU (KUM), the Helmholtz Centre Munich and the University of Bonn as well as University of Bielefeld. The German Ministry for Education and Research (Project No: 01KI20271) supported the project as did the Medical Biodefense Research Program of the Bundeswehr Medical Service. For this project, Euroimmun, Roche Diagnostics, Mikrogen, and Viramed provided kits and machines for analyses at discounted rates.
Footnotes
References 8 and 10 are preprint/non peer reviewed material
References 8 and 10 are preprint/non peer reviewed material
References 8 and 10 are preprint/non peer reviewed material mmc1.dcox
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2021.103502.
Appendix. Supplementary materials
References
- 1.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3) doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y.C., Kuo R.L., Shih S.R. COVID-19: the first documented coronavirus pandemic in history. Biomed J. 2020;43(4):328–333. doi: 10.1016/j.bj.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. WHO Coronavirus (COVID-19) Dashboard 2021, June 16 [Available from: https://covid19.who.int/.2021
- 4.Lai C.C., Wang C.Y., Ko W.C., Hsueh PR. In vitro diagnostics of coronavirus disease 2019: technologies and application. J Microbiol Immunol Infect. 2021;54(2):164–174. doi: 10.1016/j.jmii.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu S.T.H., Lin H.M., Baine I., Wajnberg A., Gumprecht J.P., Rahman F. Convalescent plasma treatment of severe COVID-19: a propensity score–matched control study. Nat Med. 2020;26(11):1708–1713. doi: 10.1038/s41591-020-1088-9. [DOI] [PubMed] [Google Scholar]
- 6.Mulchandani R., Brown B., Brooks T., Semper A., Machin N., Linley E. Use of dried blood spot samples for SARS-CoV-2 antibody detection using the Roche Elecsys ® high throughput immunoassay. J Clin Virol Off Publ Pan Am Soc Clin Virol. 2021;136 doi: 10.1016/j.jcv.2021.104739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polo G., Burlina A.P., Ranieri E., Colucci F., Rubert L., Pascarella A. Plasma and dried blood spot lysosphingolipids for the diagnosis of different sphingolipidoses: a comparative study. Clin Chem Lab Med. 2019;57(12):1863–1874. doi: 10.1515/cclm-2018-1301. [DOI] [PubMed] [Google Scholar]
- 8.Cassano J., Reut M., Korte W. Dried blood spots are an efficient blood sampling method for the detection of SARS-CoV-2 antibodies [version 1; peer review: 1 not approved]. F1000Research. 2020;9(1354).
- 9.Findeisen P., Stiegler H., Lopez-Calle E., Schneider T., Urlaub E., Hayer J. Clinical performance evaluation of a SARS-CoV-2 rapid antibody test for determining past exposure to SARS-CoV-2. Int J Infect Dis. 2021;103:636–641. doi: 10.1016/j.ijid.2020.11.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radon K., Bakuli A., Pütz P., Gleut R.L., Guggenbuehl Noller J.M., Olbrich L., et al. From first to second wave: follow-up of the prospective Covid-19 cohort (KoCo19) in Munich (Germany). medRxiv. 2021:2021.04.27.21256133. [DOI] [PMC free article] [PubMed]
- 11.Radon K., Saathoff E., Pritsch M., Guggenbuhl Noller J.M., Kroidl I., Olbrich L. Protocol of a population-based prospective COVID-19 cohort study Munich, Germany (KoCo19) BMC Public Health. 2020;20(1):1036. doi: 10.1186/s12889-020-09164-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubio-Acero R., Castelletti N., Fingerle V., Olbrich L., Bakuli A., Wölfel R. Search of the SARS-CoV-2 protection correlate: head-to-head comparison of two quantitative S1 assays in pre-characterized Oligo-/asymptomatic patients. Infect Dis Ther. 2021 doi: 10.1007/s40121-021-00475-x. [DOI] [PubMed] [Google Scholar]
- 13.Marchand A., Roulland I., Semence F., Ericsson M. Adaptation of Elecsys® anti-severe acute respiratory syndrome coronavirus-2 immunoassay to dried blood spots: proof of concept. Bioanalysis. 2021;13(3):161–167. doi: 10.4155/bio-2020-0318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.