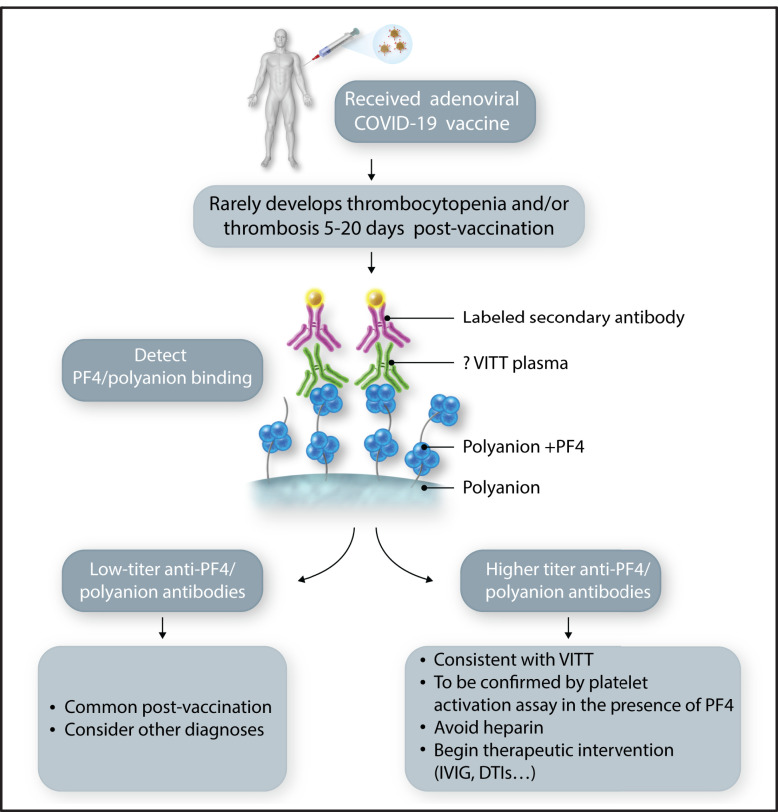
Laboratory screening for VITT. An individual receiving a vaccination for COVID-19. Those receiving an adenoviral-based vaccine have a rare risk of developing VITT. If they develop symptomatology concerning for thrombosis and/or thrombocytopenia, especially 5 to 20 days after the first vaccination, laboratory testing should be performed beginning with an antigenic anti-PF4/polyanion assay. Absent-to-low titers should be reassuring, but elevated levels should lead to a platelet functional assay and consideration of therapeutic intervention. DTI, direct thrombin inhibitor; IVIG, IV immunoglobulin. Professional illustration by Somersault18:24.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.