Table 1.
Cytotoxicity, Antiviral, Anti-IN, and Anti-RNase H Activities of Compounds 10a–w and 11a–w
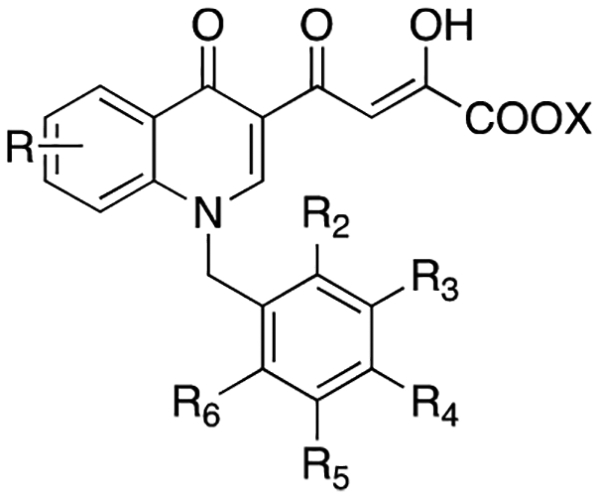 |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| activity in enzyme assays | ||||||||||||||
| IN IC50 (μM)a | RNase H | antiviral activity and cytotoxicity | ||||||||||||
| compd | R | R2 | R3 | R4 | R5 | R6 | X | 3′-P | ST | % in. at 10 μMb | IC50 (μM) | EC50 (μM)c | CC50 (μM)d | SIe |
| 10a | H | H | H | H | H | Et | >1000 | 0.70 | −3.3 | ndf | 1 | >50 | >50 | |
| 10b | F | H | H | H | H | Et | 125 | 0.28 | 2.8 | nd | 1.5 | >50 | >33.4 | |
| 10c | och3 | H | H | H | H | Et | >333 | 1.4 | 11.4 | nd | 2.6 | >50 | >19.2 | |
| 10d | H | F | H | H | H | Et | >12.3 | 4.9 | −9.4 | nd | 1.7 | >50 | >29.4 | |
| 10e | H | OCH3 | H | H | H | Et | >333 | 16 | 4.9 | nd | 8.5 | 44 | 5.2 | |
| 10f | H | H | Cl | H | H | Et | >37 | >37 | 16.1 | nd | >50 | nd | ||
| 10g | H | H | OH | H | H | Et | >333 | 1.4 | 26.0 | nd | 30.9 | >50 | >1.6 | |
| 10h | H | H | och3 | H | H | Et | >37 | >37 | 10.0 | nd | >50 | nd | ||
| 10i | H | H | NO2 | H | H | Et | >37 | >37 | 31.9 | nd | >50 | nd | ||
| 10j | F | H | F | H | H | Et | 2.2 | 3 | nd | 0.58 | >50 | >86.2 | ||
| 10k | F | H | H | F | H | Et | 3.7 | 30 | nd | >50 | nd | |||
| 10l | F | H | H | H | F | Et | >12.3 | 0.25 | 4.1 | nd | <0.2 | >50 | >250 | |
| 10m | H | F | F | H | H | Et | 5.5 | 24 | nd | 9.8 | >50 | >5.1 | ||
| 10n | H | F | H | F | H | Et | 11 | 24 | nd | 32.4 | >50 | >1.5 | ||
| 10o | Cl | H | Cl | H | H | Et | >111 | 51 | >50 | >50 | nd | |||
| 10p | Cl | H | H | H | Cl | Et | 32 | 55.0 | >50 | 30.2 | >50 | >1.7 | ||
| 10q | ch3 | H | ch3 | H | H | Et | >111 | 33 | >50 | >50 | nd | |||
| 10r | H | CH3 | H | CH3 | H | Et | 1.3 | 21 | nd | >50 | nd | |||
| 10s | F | Cl | H | H | H | Et | 1.08 | 1.08 | 21 | nd | 10 | >50 | >5 | |
| 10t | H | Cl | F | H | H | Et | 266 | 3.1 | 18 | nd | 17.4 | >50 | >2.9 | |
| 10u | 8-Cl | H | Cl | F | H | H | Et | >333 | 25.08 | 24 | nd | >50 | nd | |
| 10w | 7-Cl | F | H | H | H | H | Et | 2.8 | nd | 11 | >50 | >4.5 | ||
| 11a | H | H | H | H | H | H | 4.0 | 0.034 | 9.6 | nd | 15.5 | >50 | ||
| 11b | F | H | H | H | H | H | 1.2 | 0.016 | 45.1 | >50 | 2.6 | >50 | >19.2 | |
| 11c | OCH3 | H | H | H | H | H | >4.1 | 0.038 | 45.2 | 16.3 ± 0.5 | 9.3 | >50 | >5.4 | |
| 11d | H | F | H | H | H | H | 4.6 | 0.015 | 2.4 | nd | 28.2 | >50 | >1.8 | |
| 11e | H | och3 | H | H | H | H | >4.1 | 0.14 | 1.9 | nd | >50 | nd | ||
| 11f | H | H | Cl | H | H | H | 22 | 0.54 | 56.2 | >50 | 0.87 | >50 | >57.5 | |
| 11g | H | H | OH | H | H | H | >37 | 0.45 | 44.4 | 9.5 ± 0.4 | >50 | nd | ||
| 11h | H | H | OCH3 | H | H | H | 28 | 0.51 | 45.8 | >50 | >50 | nd | ||
| 11i | H | H | no2 | H | H | H | 27 | 0.64 | 42.4 | 47.0 ± 1.5 | >50 | nd | ||
| 11j | F | H | F | H | H | H | 0.010 | 46 | 35.9 ± 0.8 | 13.8 | >50 | >3.6 | ||
| 11k | F | H | H | F | H | H | 3.2 | 37 | >50 | >50 | nd | |||
| 11l | F | H | H | H | F | H | 0.7 | 0.019 | 57.9 | 10.8 ± 0.4 | 8.1 | >50 | >6.2 | |
| 11m | H | F | F | H | H | H | 0.11 | 44 | >50 | 16.2 | >50 | >3.1 | ||
| 11n | H | F | H | F | H | H | 0.22 | 30 | >50 | >50 | nd | |||
| 11o | Cl | H | Cl | H | H | H | 2.3 | 68 | 44.7 ± 2.0 | >50 | nd | |||
| 11p | Cl | H | H | H | Cl | H | 0.27 | 86.3 | 10.0 ± 0.3 | >50 | nd | |||
| 11q | ch3 | H | ch3 | H | H | H | 7.0 | 52 | 19.6 ± 0.6 | >50 | nd | |||
| 11r | H | ch3 | H | ch3 | H | H | 0.05 | 47 | 37.8 ± 0.9 | >50 | nd | |||
| 11s | F | Cl | H | H | H | H | 1.75 | 0.018 | 12 | nd | 23.4 | >50 | >2.1 | |
| 11t | H | Cl | F | H | H | H | 21 | 0.19 | 25 | nd | >50 | nd | ||
| 11u | 8-Cl | H | Cl | F | H | H | H | 9.8 | 0.096 | 16 | nd | 50 | nd | |
| 11v | 8-Cl | F | H | H | H | H | H | 20.5 | 0.13 | 45 | >50 | >50 | nd | |
| 11w | 7-Cl | F | H | H | H | H | H | 36.5 | 22.6 ± 0.5 | 17 | >50 | >2.9 | ||
| 1 | 12.8 ± 6g | 0.087 ± 0.008h | nd | >100 | 0.0236 ± 0.0046 | >50 | >2118 | |||||||
| EVG | 8.1 ± 4.2g | 0.028 ± 0.006g | nd | 91 ± 8 | 0.0142 ± 0.0052 | >50 | >3521 | |||||||
