Figure 3.
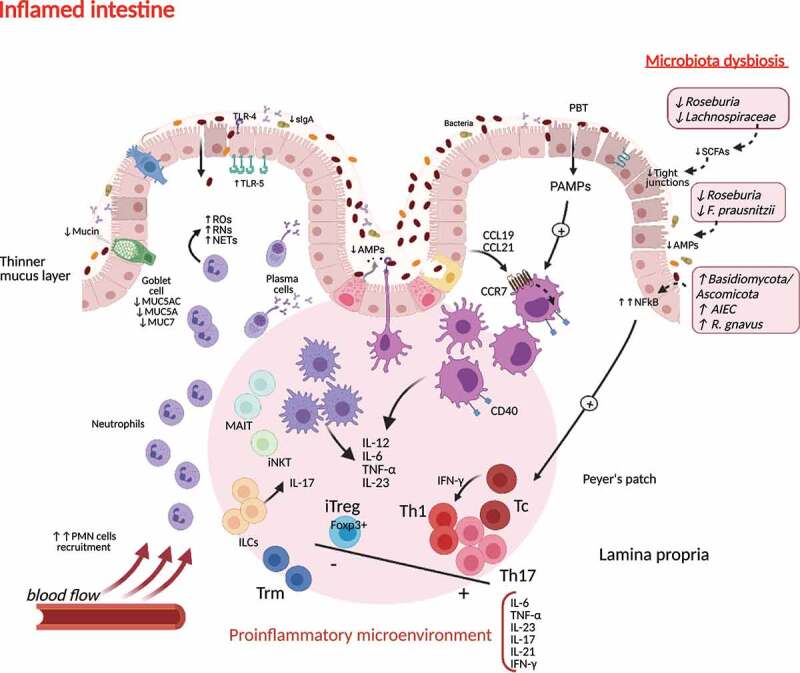
Immunogenic microenvironment during microbiota dysbiosis in inflamed intestinal tissue in Crohn’s disease. Crohn’s disease triggers induce a proinflammatory response orchestrated by tissue resident APCs activation, driving neutrophil recruitment, oxidative damage, the expansion of Th1 and Th17 populations and suppression of the regulatory T cell milieu. This environment favors the access of Tc and Trm cells to the damaged tissue. Innate lymphoid subpopulations cooperate in maintaining this immunogenic profile. Changes in gut microbiota composition and the reduction of SCFAs production contribute to the loss of tight junctions and the decrease of AMPs in the epithelial barrier, favoring an increased permeability (“leaky gut”). sIgA, soluble IgA; AMPs, antimicrobial peptides; ILC, innate lymphoid cells; MAIT, mucosal associated invariant T cell; iNKT, invariant Natural Killer T cell; DC, dendritic cell; iTreg, induced regulatory T cells; NFkB, nuclear factor kappa-B; Th, T helper cell; SCFAs, short-chain fatty acids, PMN, polymorphonuclear cells; TLRs, Toll-like receptors; Tc, T cytotoxic cell; NLRs, Nod-like receptors; IL, interleukin; ROs, reactive oxygen species; RNs, reactive nitrogen species; NETs, neutrophil extracellular traps; TNF-α, tumor necrosis factor-α; IFN-γ, interferon-gamma; CCL, chemokine ligand; CCR, chemokine receptor; PBT, pathological bacterial translocation; PAMPs; pathogen-associated molecular patterns; AIEC, adherent invasive Escherichia coli.
