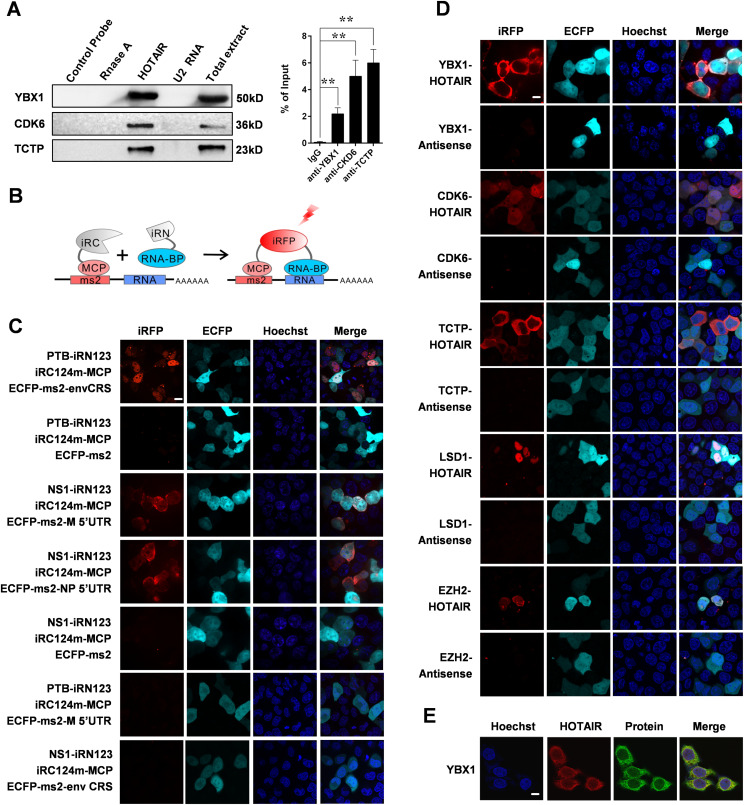
Figure 2. A Novel iRFP-TriFC System for Investigating long noncoding RNA-protein interactions.
(A) In vitro validation of interactions between HOTAIR and the indicated proteins. Left: ChIRP experiment was performed, followed by Western blotting. A nontargeting probe group (control probe), an RNase A–treated group and an unrelated RNA U2 group were used as negative controls. Right: RIP was performed to validate the RNA-protein interactions. Data are means ± SD of triplicate experiments. *P < 0.05 and **P < 0.01 (t test). (B) Schematic overview of TriFC constructs. iRN and iRC (gray segment) represent the iRFP N-terminal and C-terminal fragments, respectively. (C) Validation the iRFP-TriFC system with three known protein–RNA interaction pairs: PTB/envCRS; NS1/M 5′UTR; NS1/NP 5′UTR. ECFP-ms2 was used instead of ECFP-ms2-envCRS, ECFP-ms2-M 5′UTR, or ECFP-ms2-NP 5′UTR in the iRFP-TriFC system as a negative control. The PTB/M 5′ UTR and NS1/envCRS protein–RNA pairs were also used as negative controls. (D) In vivo confirmation of protein interactions with HOTAIR using the iRFP-TriFC approach. (E) Co-localization of HOTAIR (red) and YBX1 (green) within HeLa cells as determined by immunofluorescence (IF) and RNA-FISH. Hoechst 33342 was used for nuclear staining, and a 63×, 1.4 NA, oil immersion objective lens was used for all fluorescent image capture. Scale bars: 10 μm.