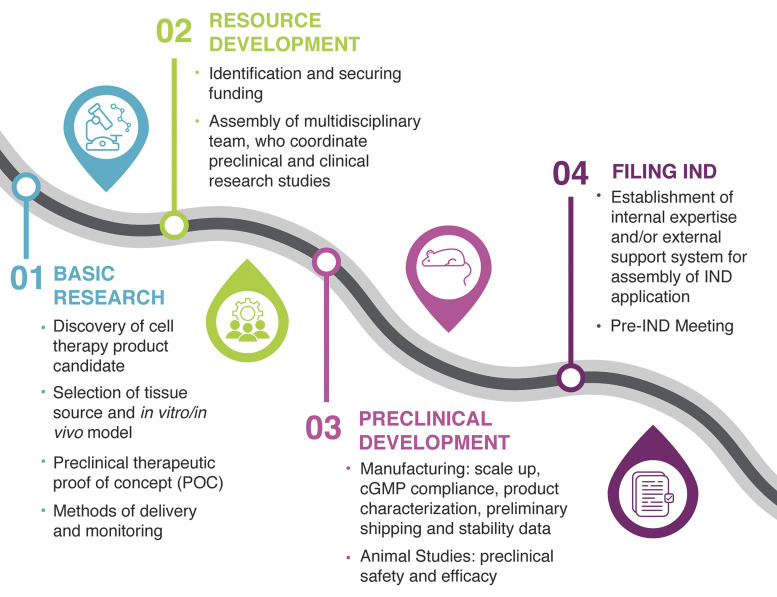
Figure 1.
Roadmap to organoid-based therapy. The roadmap identifies the four critical areas that need to be considered when developing a cellular therapy intended for evaluation in human clinical studies under an investigational new drug (IND) application: basic research, resource and preclinical development, and IND assembly and submission. POC, proof-of-concept; CGMP, current good manufacturing practice.